��Ŀ����
����Ŀ����ˮ�Ǿ�Ļ�ѧ��Դ���⣬���ú�ˮ������ȡ�ܶ����ʡ�
����1�����幤ҵ
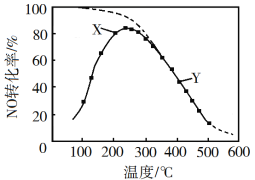
�ú�ˮɹ��֮�����±����ȡ�壬��ȡ������ͼ��
(1)���������ȿ����ܽ��嵥�ʴ�����ԭ����_____����������ͨ��ˮ�������м��ȣ���Ҫ�����¶���90�����ҵ�ԭ����______��
����2����þ��ҵ
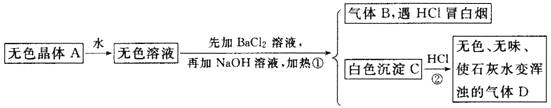
�Ӻ�ˮ��ȡʳ�κ�Br2֮�����±�г�����Mg2+��C1-�⣬����������Na+��Fe2+��Fe3+��SO42-��CO(NH2)2�ȣ�������������ȡMgCl2��MgO��Mg(OH)2�����ʣ�������ͼ��ʾ��
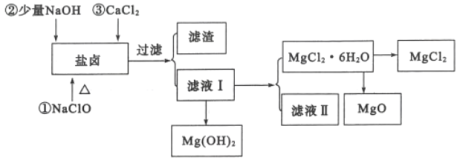
(2)��NaC1O��ȥ����CO(NH2)2ʱ������������⣬�����ܲ������ѭ���������ʣ���÷�Ӧ�Ļ�ѧ����ʽΪ_______������NaC1O����һ��������________��
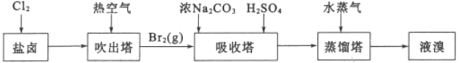
����3��������ҵ
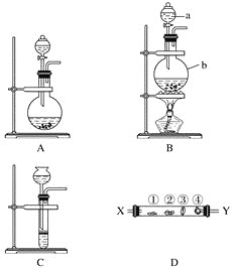
(3)��ˮ�����ķ�����Ҫ�������������ӽ����������������ȡ����ӽ�����������ˮģ�������ͼ��ʾ�����������ӽ���ԭ���ɱ�ʾΪ��HR+Na+=NaR+H+�����������������ӽ�����֬��䲿�ִ��ڵķ�Ӧ�У�ROH+C1-=RC1+OH-��______��________��
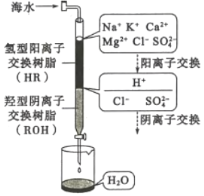
(4)����������һ���������ӽ���Ĥ���к�ˮ�����ķ�������ԭ����ͼ��ʾ��
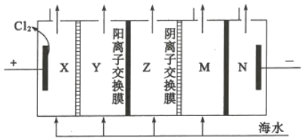
��ͼ�еĺ�ˮû��ֱ��ͨ�뵽�������У���ԭ����_________��
�ڵ���ˮ��________��(��X��Y��Z��M��N)�γɺ�������
���𰸡��嵥�ʵķе�ͣ��嵥���ӷ� �¶ȹ��Ͳ���������������¶ȹ��������϶�ˮ���� 3NaC1O+CO(NH2)2=3NaC1+CO2��+N2��+2H2O ��Fe2+����ΪFe3+�����γ�Fe(OH)3 2ROH+SO42-=R2SO4+2OH- H++OH-=H2O ��ˮ�к��н϶��Mg2+��Ca2+�������ӣ������ŵ����OH-����������Mg(OH)2��Ca(OH)2�ȳ��������ڵ缫������������Ĥ Y��M
��������
(1)����Һ���ӷ�������H2O��Һ��ķе㲻ͬ�����жϣ�
(2)NaC1O��ȥ����CO(NH2)2ʱ������������ԭ��Ӧ���������ܲ������ѭ������������CO2��N2��ͬʱ����H2O��NaCl����ϵ����غ㡢ԭ���غ���д��Ӧ����ʽ������ˮ�к��л�ԭ����Fe2+������NaC1O����ǿ�����ԡ�ˮ���Լ��Է�����
(3)��Ͻ�����������༰���������ӽ�����֬���ص������Ӧ���ɣ�
(4)�ٺ�ˮ�ǻ������к��и����Ӻ�þ���ӣ��ٽ�ϵ缫��Ӧ�������ɣ�
�ڽ�ϵ��װ�������ӵ��ƶ�����ͼʾ�����ӽ���Ĥ���ص�������ɣ�
(1)���������ȿ����ܽ��嵥�ʴ�����ԭ��������Һ��е�ͣ��ӷ�����������ͨ��ˮ�������м��ȣ���Ҫ�����¶���90�������������¶ȹ��Ͳ�����������������¶ȹ��������϶�ˮ������
(2)�����NaC1O����ǿ�����ԣ����ؾ��л�ԭ�ԣ���NaClO��ȥ����CO(NH2)2ʱ�����߷���������ԭ��Ӧ������CO2��N2��H2O��NaCl�����ݵ����غ㡢ԭ���غ㣬�ɵ÷�Ӧ����ʽΪ��3NaC1O+CO(NH2)2=3NaC1+CO2��+N2��+2H2O������ȡ�κ����±ˮ�к��л�ԭ����Fe2+����NaC1O����ǿ�����ԣ��ܹ���Fe2+��������Fe3+��ͬʱNaC1O��ǿ�������Σ�ˮ��ʹ��Һ�Լ��ԣ�Fe3+��ˮ�������OH-����γ�Fe(OH)3��������˼���NaC1O����һ�������ǽ�Fe2+����ΪFe3+�����γ�Fe(OH)3������ȥ��
(3)������֬�з������Ǹ��ֽⷴӦ�����ͼʾ��֪�����ǻ��ͽ�����֬���������ӡ������Ӻ���������ӣ������������뽻����֬��Ӧ�������������������ӻᷴӦ���漰��Ӧ�У�ROH+Cl-=RCl+OH-��2ROH+SO42-=R2SO4+2OH-��H++OH-=H2O��
(4)�ٺ�ˮΪ�������к��д�����Mg2+��Ca2+�������ӣ��������ڵ��ʱˮ�ŵ����H2ͬʱ�����OH-��OH-����Mg2+��Ca2+��Ӧ����Mg(OH)2��Ca(OH)2�ȳ��������ڵ缫������������Ĥ����˲��ܽ���ˮֱ��ͨ�뵽�����ң�
��X��Y��Z��M�о�����ĺ�ˮ�����ݵ��װ�ÿ�֪���Ϊ���ص��������Ҳ�Ϊ��������Һ�е��������������ƶ����������������ƶ����ɴ˿ɷ���Y�е�Cl-��ͨ�������ӽ���Ĥ�������X�ң������е�Na+��ͨ�������ӽ���Ĥ�����Ҳ�Z�У�ͬ��M�е�������Na+��ͨ�������ӽ���Ĥ�����Ҳ�������N�У����е�������Cl-��ͨ�������ӽ���Ĥ����Z�У���Z�е����������Ӷ�������Ӧ�Ľ���Ĥ������ܵõ���ˮ����Y��M����Z�е��Ȼ���Ũ������

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д�����Ŀ�������к͵ζ�
��1������ʽ�ζ�����ȡ20.00 mL����ϡ������Һ������ƿ�У����μ�1��2�η�̪��ָʾ������0.20 mol��L-1NaOH����Һ���еζ���Ϊ�˼�Сʵ������ͬѧһ������������ʵ�飬����ÿ����ȡϡ���������Ϊ20��00 mL������ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ |
����NaOH��Һ���/mL | 19.00 | 23.00 | 23.02 |
�������Ũ��ԼΪ_____________(������λ��Ч����)���ζ��ﵽ�յ�ı�־��_____________��
��2��������ʵ���У����в���������������ȷ������ɲⶨ���ƫ�ߵ���_______��
A. �ζ��յ����ʱ���Ӷ���
B. ��ʽ�ζ���ʹ��ǰ��ˮϴ��δ�ô���������ϴ
C. ��ƿˮϴ��δ����
D. ����NaOH����Һʱ��û�е��ܽ�Һ�������¾�ת��������ƿ��
E. ����NaOH����Һʱ������ʱ��������ƿ�Ŀ̶���
F. ��ʽ�ζ��ܼ��첿�������ݣ��ζ�����ʧ
������1���ڴ���CuSO4��5H2O�����г���������Fe2+�����ᴿʱΪ�˳�ȥFe2+�������������������ʹFe2+����ΪFe3+���������ʿɲ��õ���________
A. KMnO4 �� B. H2O2 ���� C. ��ˮ ���� D. HNO3
��2��Ȼ���ټ����ʵ����ʵ�������ҺpH=4��ʹFe3+ת��ΪFe(OH)3�����Դﵽ��ȥFe3+������ʧCuSO4��Ŀ�ģ�������ҺpH��ѡ�������е�_______��
A. NaOH B. NH3��H2O C. CuO D. Cu(OH)2
��3��������Fe(OH)3���ܶȻ�Ksp=8.0��10-38,Cu(OH)2���ܶȻ�Ksp=3.0��10-20��ͨ����Ϊ��������Һ�е�����Ũ��С��1��10-5 mol��L-1ʱ����Ϊ������ȫ������Һ��CuSO4��Ũ��Ϊ3.0 mol��L-1����Cu(OH)2��ʼ����ʱ��Һ��pHΪ_______��Fe3+��ȫ����ʱ��Һ��pHΪ________����֪ lg5 = 0.7 )
����Ŀ���״�����Ҫ�Ļ���ԭ�ϡ����úϳ���(��Ҫ�ɷ�Ϊ(CO��CO2��H2)�ڴ����������ºϳɼ״������ܷ����ķ�Ӧ���£�
i��CO2(g)+3H2(g)![]() CH3OH(g)+H2O(g) ��H1
CH3OH(g)+H2O(g) ��H1
ii��CO2(g)+H2(g)![]() CO(g)+H2O(g) ��H2
CO(g)+H2O(g) ��H2
iii��CH3OH(g)![]() CO(g)+2H2(g) ��H3
CO(g)+2H2(g) ��H3
�ش��������⣺
(1)��֪��Ӧ2����ػ�ѧ�������������£�
��ѧ�� | H��H | C=O | C��O | H��O |
E/KJ��mol-1 | 436 | 803 | 1076 | 465 |
�ɴ˼�����H2=___kJ��mol-1����֪��H1=-63kJ��mol-1������H3=___kJ��mol-1��
(2)���ڷ�Ӧ1����ͬ�¶ȶ�CO2��ƽ��ת���ʼ�������Ч��Ӱ����ͼ��ʾ����ش��������⣺
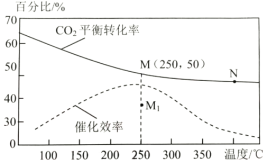
������˵������ȷ������__________��
A.M��ʱƽ�ⳣ����N��ʱƽ�ⳣ����
B.�¶ȵ���250��ʱ�����¶����״���ƽ����ʽ���
C.�����������䣬����ʹ�ô�������250��ʱCO2��ƽ��ת���ʿ���λ��M1
D.ʵ�ʷ�ӦӦ�������ڽϵ͵��¶��½��У������CO2��ת����
�����ڸ��������г���3molH2��1molCO2������Ӧ1����ʼѹǿΪ4MPa����ͼ��M��CH3OH���������Ϊ___��250��ʱ��Ӧ��ƽ�ⳣ��Kp=___(MPa)-2(������λ��Ч����)��
����Ҫ��һ����״����ʣ��ɲ�ȡ�Ĵ�ʩ��___(д��������)
(3)��ͬ�����£�һ������CO/CO2/H2�������״��������ʴ���CO2/H2�������״��������ʣ���Ϸ�Ӧ1��2����ԭ��___��
(4)�Զ������ѱ��渲��Cu2A12O4Ϊ���������Խ�CO2��CH4ֱ��ת�������ᣬCO2(g)+CH4(g)![]() CH3COOH(g)���ڲ�ͬ�¶��´����Ĵ�Ч�������������������ͼ��ʾ��
CH3COOH(g)���ڲ�ͬ�¶��´����Ĵ�Ч�������������������ͼ��ʾ��

250~300��ʱ��������������ʽ��͵���Ҫԭ����___��
300~400��ʱ������������������ߵ���Ҫԭ����___��