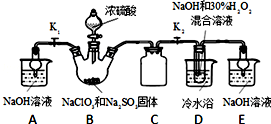
��Ŀ����
14���Ը������������Ĺ�ҵ��ҺΪԭ�������������Ĺ������£����ֲ����������ԣ����ӷ�Һ���ᴿ���ᾧ��FeSO4•7H2O
��FeSO4•7H2O���Ƴ���Һ
��FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��õ���FeCO3����Һ
��������Һ���ˣ���90����ˮϴ�ӳ����������õ�FeCO3����
��������FeCO3���õ�Fe2O3����
��֪��NH4HCO3����ˮ�зֽ⣮
��1�����������������м�Գ�ȥ��Һ�е�Fe3+���÷�Ӧ�����ӷ���ʽ��Fe+2Fe3+=3Fe2+��
��2���������һ�������ᣮ���û�ѧƽ��ԭ��������������ã��������ᣬ������Ũ������ʹ��ˮ��ƽ��Fe2++2H2O?Fe��OH��2+2H+�����ƶ����Ӷ���������������ˮ�⣮
��3����������FeCO3�����ӷ���ʽ��Fe2++2HCO3-=FeCO3��+CO2��+H2O����FeCO3��Һ��ʱ�䱩¶�ڿ����У�����
����
���ֹ�������Ϊ���ɫ���ñ仯�Ļ�ѧ����ʽ��4FeCO3+O2+6H2O=4CO2��+4Fe��OH��3��
��4����֪����FeCO3�Ļ�ѧ����ʽ��4FeCO3+O2$\frac{\underline{\;����\;}}{\;}$2Fe2O3+4CO2��������464.0g��FeCO3���õ�316.8g��Ʒ������Ʒ������ֻ��FeO����ò�Ʒ��Fe2O3��������288.0g��
���� ��1��Fe���Ժ���������Ӧ�����������ӣ�
��2��FeSO4•7H2O���Ƴ���Һ�Ĺ����У�����������ˮ�⣬����ƽ���ƶ�ԭ����������
��3���������Ӻ�̼�����������ٽ�ˮ������̼�������Ͷ�����̼�����������ױ���������Ϊ��������
��4�����ݺ���Ԫ�����ʵ�������ϵ�����㣬����Fe2O3���ʵ���Ϊx mol��FeO�����ʵ���Ϊy mol����ô���㣺160x+72y=316.8����2x+y����116=464.0������õ���
��� �⣺��1��Fe���Ժ���������Ӧ�����������ӣ���Fe+2Fe3+=3Fe2+��
�ʴ�Ϊ��Fe+2Fe3+=3Fe2+��
��2��FeSO4•7H2O���Ƴ���Һ�Ĺ����У�����������ˮ�⣬Fe2++2H2O?Fe��OH��2+2H+�������������ƿ���ʹ�û�ѧƽ�������ƶ�������ˮ�⣬
�ʴ�Ϊ���������ᣬ������Ũ������ʹ��ˮ��ƽ��Fe2++2H2O?Fe��OH��2+2H+�����ƶ����Ӷ���������������ˮ�⣻
��3��FeSO4��Һ���Թ�����NH4HCO3��Һ��ϣ��������Ӻ�̼���������˫ˮ������̼�������Ͷ�����̼����Fe2++2HCO3-=FeCO3��+CO2��+H2O�����ɵ�̼�������У����������ױ���������Ϊ����������4FeCO3+O2+6H2O=4CO2��+4Fe��OH��3��
�ʴ�Ϊ��Fe2++2HCO3-=FeCO3��+CO2��+H2O��4FeCO3+O2+6H2O=4CO2��+4Fe��OH��3��
��4������Fe2O3���ʵ���Ϊx mol��FeO�����ʵ���Ϊy mol����ô���㣺160x+72y=316.8����2x+y����116=464.0�����x=1.8mol�����Բ�����Fe2O3������160g/mol��1.8mol=288.0g=288.0g��
�ʴ�Ϊ��288.0��
���� ���⿼��ѧ����Ԫ�صIJ�ͬ��̬֮���ת�������Ը�����ѧ֪ʶ���лش����ջ����ǹؼ�����Ŀ�ѶȽϴ�

 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�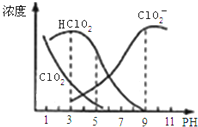 ����������һ�ָ�Ч������Ư������Ҫ�����ġ����顢ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з�����ȷ���ǣ�������
����������һ�ָ�Ч������Ư������Ҫ�����ġ����顢ֽ����Ư�ף��������ƣ�NaClO2������Һ�п�����ClO2��HClO2��ClO2-��Cl-�ȣ�����HClO2��ClO2������Ư�����ã���ClO2���ж����壮���ⶨ��25��ʱ����ֺ�����pH�仯�����ͼ��ʾ��Cl-û�л������������з�����ȷ���ǣ�������| A�� | �������������������½��ȶ� | |
| B�� | 25��ʱ��HClO2�ĵ���ƽ�ⳣ������ֵKa=10-6 | |
| C�� | pHԽ��Ư����Ư������Խ�� | |
| D�� | 25�棬pH=3ʱ��NaClO2��Һ�У�c��Na+��+c��H+��=c��ClO2-��+c��OH-�� |
| A�� | ��CO2��O2��ɵĻ�����й���NA�����ӣ����е���ԭ����Ϊ2NA | |
| B�� | ��״���£�14 g�������еĺ��������Ϊ5NA | |
| C�� | ��״���£�5.6 L���Ȼ�̼��CCl4�����еķ�����Ϊ0.25NA | |
| D�� | NA��һ����̼���Ӻ�0.5 mol �����������Ϊ7��4 |
| A�� | 1 mol/L | B�� | 0.1mol/L | C�� | 0.001mol/L | D�� | 10mol/L |
| A�� | ��ϡ����ϴ����������Ӧ���Թܣ�3Ag+4H++NO3-=3Ag++NO��+2H2O | |
| B�� | ������������Һ��ȥ�����������Ĥ��Al��OH��3+OH-=AlO2-+2H2O | |
| C�� | ��˫��ˮ��ϡ���ᴦ��ӡˢ��·�壺Cu+H2O2+2H+=Cu2++2H2O | |
| D�� | ��ʳ�׳�ȥˮƿ�е�ˮ����CO32-+2CH3COOH=2CH3COO-+CO2��+H2O |