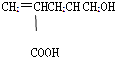��Ŀ����
����Ŀ��ij��Һ��ֻ���ܺ���K+��NH4+��Fe2+��Al3+��Cl-��SO42-��CO32-��AlO2-�е����������ӡ�����Ũ�Ⱦ�Ϊ0.3mol��L-1��ijͬѧ����������ʵ�飺

����˵����ȷ����
A. ��ȷ��ԭ��Һ���Ƿ���Al3+��Cl-
B. ԭ��Һ�д��ڵ�����ΪNH4+��Fe2+��Cl-��SO42-
C. ��ҺX�д������ڵ���������NH4+��Fe2+��Ba2+
D. ��ȷ������C�ijɷ�
���𰸡�B
������������Һ����ϡ��������������˵����Һ������CO32-��AlO2-������Ba(NO3)2��Һ������������NO��˵����Һ���л�ԭ������Fe2+����������������Һ�������壬˵����Һ����NH4+��A. ��ʵ��õ����ۣ���Һ����Fe2+��NH4+��������CO32-��AlO2-��Ϊ�˱��ֵ���غ㣬Ҫ����Һ����Cl-��SO42-��������Al3+����A����B. ԭ��Һ�д��ڵ�����ΪNH4+��Fe2+��Cl-��SO42-����B��ȷ��C. �ڹ���ϡ��������ᱵ��Һ�����£���ҺX��Fe2+���ܴ������ڣ���C����D. ����C�ijɷ���̼�ᱵ����D����ѡB��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�����Ŀ�����ΪԪ�����ڱ���һ���֣�
̼ | �� | Y | |
X | �� | Z |
�ش��������⣺
��1��ZԪ�������ڱ��е�λ��Ϊ ��
��2������Ԫ��ԭ�Ӱ뾶�����ǣ���Ԫ�ط��ţ� ��
��3��������ʵ��˵��YԪ�صķǽ����Ա�SԪ�صķǽ�����ǿ���� �� a��Y������H2S��Һ��Ӧ����Һ�����
b����������ԭ��Ӧ�У�1molY���ʱ�1molS�õ��Ӷ�
c��Y��S��Ԫ�صļ��⻯�����ȷֽ⣬ǰ�ߵķֽ��¶ȸ�
��4���е㣺H2YH2S���������=���������������� ��
��5��Y2��Y3��Ϊ���ͬλ�ء���ͬ���칹�塱��ͬ�������塱����
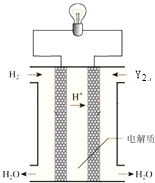
��6�����е���Y2��������ͼ��ʾװ�ã�ȼ�ϵ�أ�������������д��Y2�����Ե������Һ�з����ĵ缫��Ӧ����ʽ ��