��Ŀ����
Ϊ�ⶨijH2C2O4��Һ��Ũ�ȣ�ȡ25��00mL����Һ����ƿ�У���������ϡH2SO4����Ũ��Ϊc mol/L KMnO4����Һ�ζ����ζ�ԭ��Ϊ��
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
��1���ζ�ʱ��KMnO4��ҺӦװ�� �����ʽ�ζ��ܡ���ʽ�ζ��ܡ� ���У��ﵽ�ζ��յ������Ϊ ��
��2�����ζ�ʱ��û�ñ�Һϴ�ӵζ��ܣ���ʹ�ò�����Һ�����ʵ���Ũ��_ _ (�ƫ�ߡ���ƫ�͡�����Ӱ�족)
��3�����ζ�ʱ����Ӧǰ������ζ����ֱ�Ϊa��b����ʵ�������������Һ�����ʵ���Ũ��Ϊ mol/L��
��4���ڸ���Һ��KOH��Һ��Ӧ���õ�0��1 mol/L KHC2O4��Һ�У�c(C2O42-)��c��H2C2O4�������й�ϵ��ȷ���� ��
| A��c��K+��+c��H+��=c(HC2O4-)+c(OH-)+c(C2O42-) |
| B��c(HC2O4-)+ c (C2O42-)+ c��H2C2O4��=0��1mol/L |
| C��c(H+)��c��OH-�� |
| D��c��K+��=c��H2C2O4��+c(HC2O4-)+c(C2O42-) |
��1����ʽ�ζ��� (2��) �μ����һ��KMnO4����ƿ����Һ����ɫͻ��Ϊ�Ϻ�ɫ���Ұ��������ɫ���ָ��� (2��)
��2��ƫ�� (2��)
��3��0��1c(b-a) (3��)
��4��BD (3��)
�������������1����ΪKMnO4����ǿ�����ԣ��ḯʴ�ܣ���Ӧ����ʽ�ζ���ʢװ���ʴ�Ϊ��ʽ�ζ��ܡ���KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KnO4��Һʱ����Һ������ɫ��Ϊ��ɫ���ʴ�Ϊ�����������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ㣻��2�����ζ�ʱ��û�ñ�Һϴ�ӵζ��ܣ��൱�ڰѱ���Һ������ϡ�ͣ��������ĵĵı�Һ���ƫ�ߣ���ʹ�ò�����Һ�����ʵ���Ũ��ƫ�ߡ���3�����ζ�ʱ����Ӧǰ������ζ����ֱ�Ϊa��b�����Һ�����Ϊ(b-a)ml����ñ�ҺKMnO4�����ʵ���Ϊ��c(b-a)/1000mol������2KMnO4-----5H2C2O4��ϵʽ��������������Һ�����ʵ���Ũ��Ϊ0��1c(b-a)mol/L����4����c(C2O42-)��c��H2C2O4����˵��HC2O4-����̶ȴ���ˮ��̶ȡ� A�� c��K+��+c��H+��=c(HC2O4-)+c(OH-)+c(C2O42-)����غ��ϵʽ��д���� B��c (HC2O4-)+ c (C2O42-)+ c��H2C2O4��=0��1mol/L�������غ㣬��ȷ�� HC2O4-����̶ȴ���ˮ��̶ȣ��� C�� c(H+)��c��OH-������ D��c��K+��=c��H2C2O4��+c(HC2O4-)+c(C2O42-)���غ����ʽ����ȷ��
���㣺���⿼�����к͵ζ�ʵ�飬�Ѷ����У�ע�����ղ��Ậ���ļ��㷽�����к͵ζ��е����������ɽ��

 ��Ӣ���㿨ϵ�д�
��Ӣ���㿨ϵ�д� Ӧ����㲦ϵ�д�
Ӧ����㲦ϵ�д���֪ij��Һ��ֻ����OH����H+��Na+��CH3COO���������ӣ�ijͬѧ�Ʋ����Һ�и�����Ũ�ȴ�С˳��������������ֹ�ϵ��
| A��c (CH3COO��)��c (Na+)��c (H+)��c (OH��) | B��c (CH3COO��)��c (Na +)��c (OH��)��c (H+) |
| C��c (CH3COO��)��c (H+)��c (Na+)��c (OH��) | D��c (Na+)��c(CH3COO��)��c (OH��)��c (H+) |
��2��������Һֻ��һ�����ʣ���������Ũ�ȴ�С˳���ϵ����ȷ���ǣ�ѡ����ţ� ��
��3����������ϵ��C����ȷ�ģ�����Һ�����ʵĻ�ѧʽ�� ��
��4��������Һ�������ȵĴ����NaOH��Һ��϶��ɣ���ǡ�ó����ԣ���
���ǰc��CH3COOH�� c��NaOH�����>������<������=������ͬ����
��Ϻ���Һ��c��Na+�� c��CH3COO������
ʵ����ģ�����ij�Ͼɺ�����������Ҫ�ɷ�ΪNiO������Fe2O3��CaO��CuO��BaO�ȣ�����Ni2O3���乤������Ϊ��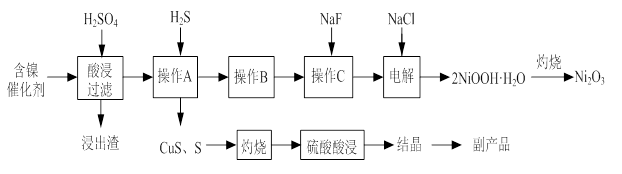
ͼ�� ͼ��
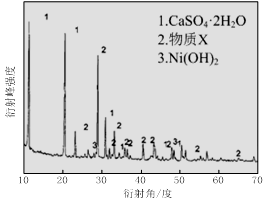
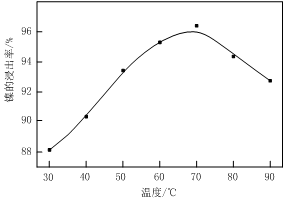
��1������ͼ����ʾ��X��������ͼ�ף���֪����������������Ҫ�ɷ֣����С�����X��Ϊ ��ͼ���ʾ���Ľ��������¶ȵĹ�ϵ���������¶ȸ���70��ʱ�����Ľ����ʽ��ͣ���������Ni(OH)2����������ԭ���� ��
��2�����������С�����Ʒ���Ļ�ѧʽΪ ��
��3����֪�й��������↑ʼ�����ͳ�����ȫ��pH���±���
| �������� | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
| ��ʼ������pH | 1.5 | 6.5 | 7.7 |
| ������ȫ��pH | 3.7 | 9.7 | 9.2 |
����B��Ϊ�˳�ȥ��Һ�е���Ԫ�أ�ijͬѧ���������ʵ�鷽���������A���õ���Һ�м���NaOH��Һ��������ҺpHΪ3.7��7.7�����ã����ˡ���Ը�ʵ�鷽���������ۣ� ����ԭ������ȷ����˵�����ɣ���ԭ������������Ը�������
��4������C��Ϊ�˳�ȥ��Һ�е�Ca2+����������Һ��F��Ũ��Ϊ3��10��3 mol��L��1����Ca2+��Ũ��Ϊ ______mol��L��1��������ʱCaF2���ܶȻ�����Ϊ2.7��10��11��
��5��������2NiOOH��H2O��ԭ�����������ټ���������Cl��������������ΪClO������Ni2+��ClO����������2NiOOH��H2O�������ڢڲ���Ӧ�����ӷ���ʽΪ ��
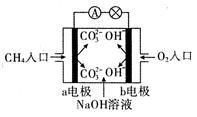
 CH3COO-+H+ ��H��0��
CH3COO-+H+ ��H��0��