��Ŀ����
4����1����֪��Ԫ��H2A��ˮ�д������µ��룺H2A�TH++HA-��HA-?H++A2-���Իش��������⣺NaHA��Һ�����ԣ�������HA-ֻ���룬������ˮ�⣮��2��ij�¶��£���10mL��0.1mol/L NaHA��Һ�м���0.1mol/L KOH��ҺV mL�����ԣ���ʱ��Һ�����¹�ϵһ����ȷ����BD����д��ĸ����
A����ҺpH=7 B��ˮ�����ӻ�KW=c2��OH-��
C��V=10 D��c��K+����c��Na+��
��3����֪0.1mol/L��NaHA��Һ��pH=2����0.1mol/L��H2A��Һ��H+��Ũ�ȣ�0.11mol•L-1���������=����������ԭ����HA-���ֵ�����H2A��һ������������������Ƶڶ������룮
���� ��1����HA-?H++A2-��֪��Na2AΪǿ�������Σ�NaHAΪ��ʽ�Σ�H2A��һ����ȫ���룬����HA-ֻ���룬������ˮ�⣻
��2��A�������¶��ж���Һ��pH��
B������c��OH-��=c��H+����
C��HA-�� OH-ǡ�÷�Ӧʱ����A2-����Һ�ʼ��ԣ���֪��ҺΪ���ԣ�˵��NaHA��Һ��ʣ�࣬��V��10
D������Cѡ���жϣ�
��3�����ݵ��뷽��ʽ֪��HA-ֻ���벻ˮ�⣬0.1mol•L-1NaHA��Һ��pH=2����HA-�����������Ũ��Ϊ0.01mol/L��H2A��һ������������������Ƶڶ������룮
��� �⣺��1����HA-?H++A2-��֪��Na2AΪǿ�������Σ�NaHAΪ��ʽ�Σ�H2A��һ����ȫ���룬����HA-ֻ���룬������ˮ�⣬HA-�������������ӣ�������Һ�����ԣ�
�ʴ�Ϊ���HA-ֻ���룬������ˮ�⣻
��2��A�������¶Ȳ�֪����������ʱ��ҺpH����ȷ������A����
B������c��OH-��=c��H+����Kw=c��OH-��•c��H+��=KW=c2��OH-������B��ȷ��
C��HA-�� OH-ǡ�÷�Ӧʱ����A2-����Һ�ʼ��ԣ���֪��ҺΪ���ԣ�˵��NaHA��Һ��ʣ�࣬��V��10����C����
D������Cѡ���жϣ�NaHA����������c��K+����c��Na+������D��ȷ��
�ʴ�Ϊ��BD��
��3�����ݵ��뷽��ʽ֪��HA-ֻ���벻ˮ�⣬0.1mol•L-1NaHA��Һ��pH=2����HA-�����������Ũ��Ϊ0.01mol/L��H2A��һ����ȫ��������0.1mol/L�������ӣ���һ������������������Ƶڶ������룬���Եڶ����������������Ũ��С��0.01mol/L����H2A��Һ�������ӵ����ʵ���Ũ��ӦС��0.11mol/L��
�ʴ�Ϊ������HA-���ֵ�����H2A��һ������������������Ƶڶ������룮
���� ���⿼��������ʵĵ��룬ע�������Ϣ��H2A���������벻ͬ����һ����ȫ���롢�ڶ������ֵ��룬����HA-ֻ���벻ˮ�⣬Ϊ�״��⣮

| A�� | Al3+��Cl-��Ca2+ | B�� | Mg2+��SO42-��OH- | C�� | Na+��SO32-��H+ | D�� | H+��K+��OH- |
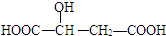 ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������| A�� | ƻ�������ܷ���������Ӧ�Ĺ�������2�� | |
| B�� | 1molƻ�������3mol NaOH�����кͷ�Ӧ | |
| C�� | 1molƻ��������������Na��Ӧ��������1molH2 | |
| D�� | HOOC-CH2-CH��OH��-COOH��ƻ���ụΪͬ���칹�� |
| A�� | ��Ԫ�صĵ����ڳ����¸�ˮ��Ӧ�����ƾ��� | |
| B�� | ��Ԫ�ص�ԭ�Ӱ뾶�ȼص�ԭ�Ӱ뾶С | |
| C�� | ��Ԫ�ص�̼����������ˮ | |
| D�� | ��Ԫ������������ˮ������ʹAl��OH��3�ܽ� |
| A�� | pH=7��NaHSO3��Na2SO3�����Һ�У�c��Na+��=c��HSO3-��+c��SO32-�� | |
| B�� | �����ʵ���Ũ�ȵ�������Һ�У���NH4Al��SO4��2 ��NH4Cl ��CH3COONH4��NH3•H2O��c��NH4+���ɴ�С��˳���Ǣ٣��ڣ��ۣ��� | |
| C�� | 0.1 mol•L-1�Ĵ����pH=a��0.01 mol•L-1�Ĵ����pH=b����a+1=b | |
| D�� | 0.1 mol•L-1�Ĵ�������Һ20 mL��0.1 mol•L-1������10 mL��Ϻ���Һ�����ԣ����У�c��CH3COOH����c��Cl-����c��CH3COO-����c��H+����c��OH-�� |
| A�� | ��A���Ԫ��ȫ�����ǽ���Ԫ�� | |
| B�� | Ԫ�����ڱ���18�����У�����18���� | |
| C�� | ͬ�����е�IA��Ԫ�صĽ����ԱȢ�A��Ԫ�صĽ�����ǿ | |
| D�� | Ԫ�����ڱ������Ϸ������Ԫ�ض���������뵼����� |
 �Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺
�Ѻ��ѵĺϽ��ѱ��㷺���������Ѷ���ġ���������������豸���ɻ��Ⱥ��캽�ղ��ϣ�����Ϊ��δ������Ľ��������Իش��������⣺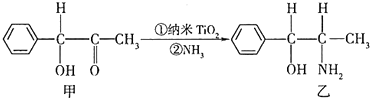
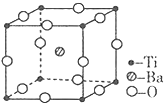
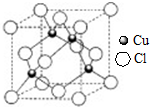 ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�������������Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29���ش��������⣺
ԭ������С��36��X��Y��Z��W����Ԫ�أ�����X���γɻ�������������Ԫ�أ�Yԭ�ӻ�̬ʱ���������������ڲ��������2����Zԭ�ӻ�̬ʱ2pԭ�ӹ������3��δ�ɶԵĵ��ӣ�W��ԭ������Ϊ29���ش��������⣺