��Ŀ����
����Ŀ��[��ѧ����ѡ��3�����ʽṹ������]̼���仯����㷺��������Ȼ���У��ش��������⣺
��1������һ���ռ��˶�״̬�ĵ�����ԭ�Ӻ�����ֵĸ����ܶȷֲ�����___________�����������ڻ�̬ԭ���У��������______�������෴�ĵ��ӡ�
��2��̼���γɻ�����ʱ��������Թ��ۼ�Ϊ����ԭ����_____________��
��3��CS2�����У����ۼ���������_____________��Cԭ�ӵ��ӻ����������_______��д��������CS2������ͬ�ռ乹�ͺͼ�����ʽ�ķ��ӻ�����_______________��
��4��CO�������Fe�γ�Fe(CO)5���û�������۵�Ϊ253K���е�Ϊ376K�����������_____���塣
̼�ж���ͬ�������壬����ʯīϩ����ʯ�ľ���ṹ��ͼ��ʾ��
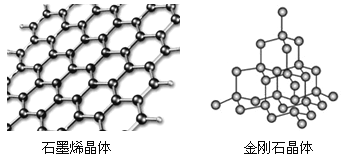
����ʯīϩ�����У�ÿ��Cԭ������_____����Ԫ����ÿ����Ԫ��ռ��___��Cԭ�ӡ�
���ڽ��ʯ�����У�Cԭ�������ӵ���С��ҲΪ��Ԫ����ÿ��Cԭ������________����Ԫ������Ԫ���������________��Cԭ����ͬһƽ�档
���𰸡������� 2 C��4���۵����Ұ뾶��С������ͨ���û�ʧ���Ӵﵽ�ȶ����ӽṹ ���������� sp CO2��SCN- ���� 3 2 12 4
��������
��1���������Ǵ���һ���ռ��˶�״̬�ĵ�����ԭ�Ӻ�����ֵĸ����ܶȷֲ�������������Cԭ�ӵĺ�����6�����ӣ������Ų�Ϊ1s22s22p2������1s��2s�ϵ�2�Ե��ӵ����������෴����2p����ĵ��ӵ�����������ͬ��
��2����ԭ�ӽṹ�У���������С��4����ԭ����ʧȥ���ӣ���Cԭ�ӵ��������4�����ӣ���Cԭ�ӵİ뾶��С��������ͨ���û�ʧ���Ӵﵽ�ȶ��ṹ��������Ҫͨ�����õ��ӶԼ��γɹ��ۼ��ķ�ʽ���ﵽ�ȶ��ṹ��
��3��CS2�����У�C��Sԭ���γ�˫����ÿ��˫�����Ǻ���1��������1�����������ӿռ乹��Ϊֱ���ͣ����еĹ��ۼ�����Ϊ������������Cԭ�ӵ�������γ�2���������¶Ե��ӣ�����Ϊsp�ӻ���O��Sͬ���壬������CS2������ͬ�ռ乹�ͺͼ�����ʽ�ķ���ΪCO2���������̼��Ϊ�ȵ������������SCN-������SCN-�Ŀռ乹������Ϸ�ʽ��CS2��ͬ��
��4���û������۵�Ϊ253K���е�Ϊ376K��˵���۷е�ϵͣ�����Ϊ���Ӿ��壻
��5�����ݾ�̯�������㡣��ʯīϩ�����У�ÿ��Cԭ�ӱ�3��6Ԫ�����У�ÿ����Ԫ��ռ�е�Cԭ������6��![]() = 2��
= 2��
�� ÿ��Cԭ����Χ�γ�4�����ۼ���������������ǣ�ÿ��������Ϊ������Ԫ�������ÿ��̼ԭ��������2��6=12����Ԫ����
�������ɵ���Ԫ�����д�ʽ����ʽ���ֹ���ʽ����Ĵ����ĸ�ԭ���ǹ���ģ���ʽ���������岿�ֵ��ĸ�ԭ���ǹ���ģ�������Ԫ���������4��Cԭ�ӹ��档

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���¶�ΪTʱ���������ݻ���Ϊ1L�ĺ����ܱ������н�������Ӧ��2SO2(g)��O2(g)![]() 2SO3(g) ��H��0���ﵽƽ��ʱ������˵������ȷ����
2SO3(g) ��H��0���ﵽƽ��ʱ������˵������ȷ����
���� ��� | �������� | ��ʼ���ʵ��� / mol | ƽ��ʱSO3�����ʵ��� / mol | ||
SO2 | O2 | SO3 | |||
I | ���º��� | 2 | 1 | 0 | 1.8 |
II | ���º�ѹ | 2 | 1 | 0 | a |
III | ���Ⱥ��� | 0 | 0 | 2 | b |
A. ����I��SO2��ת����С������II��SO2��ת����
B. ����II��ƽ�ⳣ����������III�е�ƽ�ⳣ��
C. ƽ��ʱSO3�����ʵ�����a��1.8��b��1.8
D. ����ʼʱ������I�г���0.10 mol SO2(g)��0.20mol O2(g)��2.0 mol SO3(g)�����ʱv����v��