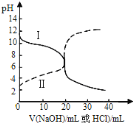
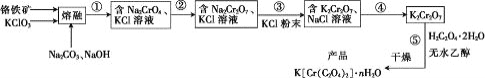
��Ŀ����
����Ŀ����.�����ϣ���ʦ������μ���Ũ������ϡ���ᣬijѧϰС����Ʒ�������
���� | ���� | |
�� | ������Ͷ���� | �����ΪŨ���� |
�� | ������Ƭ | �����̼�����ζ��ΪŨ���� |
�� | ����������ʢˮ��С�ձ��� | ������ΪŨ���� |
�� | �ò�����պŨ��ˮ�������ƿ�� | ð������ΪŨ���� |
�� | ����μӵ����������� | �����ΪŨ���� |
(1)���Ϸ����У����е���______________������ţ�
(2)����һ�������Ľ����ܳ�Ϊ���з�����________________���Ľ�����Ϊ____________��
(3)��ȫ�������__________����Ϊ_________________________________________��
��.ʵ������Ũ��������1.0mol/L������Һ480mL���ش��������⣺
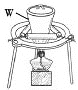
(1)����ͼ��ʾ��������������Һ�϶�����Ҫ����____________������ĸ��������������Һ����Ҫ�õ��IJ���������_________________________�����������ƣ���
(2)����ƿ�ϱ�������5���е�_____________������ţ�
��ѹǿ ���¶� ������ ��Ũ�� �ݿ̶���
(3)�����ƹ����У����в���ʹ������ҺŨ��ƫ�����______________������ţ���
��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ��
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶���
�۶���ʱ�����ӿ̶���
��ʹ������ƿǰ������ˮϴ����û����
(4)����ʵ��������������Һ������Ͳȡ��������Ϊ98%���ܶ�Ϊ1.84g/mL��Ũ��������Ϊ_____________________mL��������С�����һλ��
���𰸡��٢ۢ� �� ������ų�����ʹ��Ƭ�ܽ����ϡ���� �� �������ѻӷ����ᣨ���߸߷е��� �� AC �ձ��������� �ڢۢ� �� 36.8
��������
��.����ϡ�����Ũ�������ʲ�ͬ���ʵ�飻
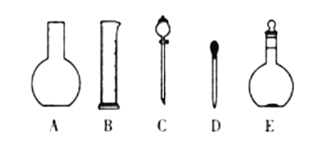
��. (1)��������һ�����ʵ���Ũ����Һ�õ�������ѡ����������Һ�����ѡ������ƿ�Ĺ��
(2)��������ƿ������
(3) �������������ʵ����ʵ�������Һ�����Ӱ�죬����C= ![]() ������������
������������
(4)����c= ![]() ����Ũ��������ʵ���Ũ�ȣ�������Һϡ�����ʵ����ʵ������������ҪŨ���������
����Ũ��������ʵ���Ũ�ȣ�������Һϡ�����ʵ����ʵ������������ҪŨ���������
(1)Ũ���������ˮ�ԣ���ʹ���ڣ�ϡ����û����ˮ�ԣ��ʢٿ��У�Al��ϡ���ᷴӦ����������Ũ�����Al�����ۻ����ʢڲ����У�Ũ�����ϡ����ϡ�����ж��ų��ȣ���Ũ����ų�������ԶԶ����ϡ���ᣬ�ʢۿ��У�Ũ�����ϡ���ᶼû�лӷ��ԣ������ò�����պŨ��ˮ����ʢ�����ƿ�ڲ��������̣��ʢܲ����У�Ũ���������ˮ�ԣ�ϡ����û����ˮ�ԣ�����Ũ���ᡢϡ����ֱ�ӵ����������ϣ������Ũ���ᣬ�ʢݿ��У�
���е��Ǣ٢ۢݣ�
(2) ����һ�������Ľ����ܳ�Ϊ���з����Ǣڣ��������ܺ�ϡ���ᷴӦ��������������Ũ����ۻ������Ե���һ�£�������Ƭ��������ų�����ʹ��Ƭ�ܽ����ϡ���
(3)��Ũ����߷е㣬���ӷ������Դ��Լ�ƿ��������ð���̣��ʢ���ȫ����
��. (1)����һ�����ʵ���Ũ����Һ�õ������У�������ƽ��ҩ�ס��ձ���Ͳ����������������ƿ����ͷ�ιܣ�����Ҫ����������ƿ�ͷ�Һ©����Ҫ����450mL��ҺӦѡ��500mL����ƿ�����Ի�ȱ�ٵ��������ձ������������ʴ�Ϊ��AC���ձ�����������
(2)����ƿ�ϱ��У��¶ȡ��������̶��ߣ���ѡ���٢ۢݣ�
(3)��δ��ϡ�ͺ��H2SO4��Һ��ȴ�����¾�ת�Ƶ�����ƿ�У���ȴ����Һ���ƫС�����ʵ����ʵ���Ũ��ƫ�ߣ���ѡ��
�ڶ���ҡ�Ⱥ���Һ����ڿ̶��ߣ����ý�ͷ�ιܼ�����ˮ���̶��ߣ�������Һ���ƫ�����ʵ����ʵ���ƫС����ҺŨ��ƫ�ͣ��ʲ�ѡ��
�۶���ʱ�����ӿ̶��ߣ�������Һ���ƫ����ҺŨ��ƫ�ͣ��ʲ�ѡ��
��ʹ������ƿǰ������ˮϴ����û�����ʵ����û��Ӱ�죬�ʲ�ѡ��
��ȷ���Ǣ٣�
(4)��������Ϊ98%���ܶ�Ϊ1.84g/mL��Ũ��������ʵ���Ũ��c=![]() =18.4mol/L������ҪŨ�������ΪV������1.0mol/L������Һ480mL��Ҫ��500mL����ƿ����������Һϡ�����ʵ����ʵ����������ã�18.4mol/L��V=1mol/L��500mL�����V=36.8mL��
=18.4mol/L������ҪŨ�������ΪV������1.0mol/L������Һ480mL��Ҫ��500mL����ƿ����������Һϡ�����ʵ����ʵ����������ã�18.4mol/L��V=1mol/L��500mL�����V=36.8mL��