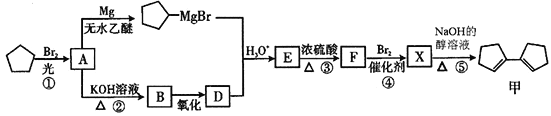
��Ŀ����
����Ŀ����ʽ�Ȼ�þ(MgOHCl)�������������Ӽ�����ҵ���Ʊ������϶࣬��������������þ�ȷֽ��Ȼ���ư������õ���ʽ�Ȼ�þ�Ĺ��������ҹ��״���ij��ѧ����С����ݸ�ԭ���������װ��ͼ�������ʵ�飬װ��C��CuO������Ϊ8.0 g��

��ش��������⣺
��1��װ��A�з�����Ӧ���ɼ�ʽ�Ȼ�þ�Ļ�ѧ����ʽΪ��_____________________________��
��2��װ��D�����ɳ�����������Ӧ�����ӷ���ʽΪ_________________________________��
��3����Ӧ�����г���ͨ��N2�����������㣺һ��: ��װ��A�в����İ�����ȫ����������:_______________________________��
��4������ü�ʯ�ҵ�����������a g����õ���ʽ�Ȼ�þ������Ϊ_______g��
��5����Ӧ��ϣ�װ��C�е�����ͭȫ���ɺ�ɫ��Ϊ��ɫ����������Ϊ6.8 g�������ɵ������ֱ���ŷŵ������У����ɫ������_______���÷�Ӧ��ת�Ƶ��ӵ����ʵ���Ϊ_______mol��
��6���������һ��ʵ�鷽��֤��װ��C�е�����ͭ��Ӧ��ȫ��õ��ĺ�ɫ�����к���������ͭ����֪����Cu2O��2H��===Cu2����Cu��H2O
����ѡ�Լ���2 mol��L��1H2SO4��Һ��Ũ���ᡢ2 mol��L��1HNO3��Һ��10 mol��L��1 HNO3��Һ
ʵ�鲽�� | Ԥ������ͽ��� |
����1��ȡ��Ӧ��װ��C�е������������Թ��� | |
����2��____________ | ____________ |
���𰸡�Mg(OH)2��NH4Cl![]() MgOHCl��NH3����H2O Al3����3NH3��H2O=Al(OH)3����3NH ϡ�Ͱ�������ֹ���� 4.25a Cu��Cu2O 0.15 ���Թ��м�������2 mol��L��1H2SO4��Һ ��Һ�����ɫ��˵����ɫ�����к���Cu2O
MgOHCl��NH3����H2O Al3����3NH3��H2O=Al(OH)3����3NH ϡ�Ͱ�������ֹ���� 4.25a Cu��Cu2O 0.15 ���Թ��м�������2 mol��L��1H2SO4��Һ ��Һ�����ɫ��˵����ɫ�����к���Cu2O
��������
A�з�Ӧ�õ�MgOHCl��������NH3��H2O����ʯ�Ҹ��ﰱ����C�а���������ͭ��Ӧ��õ�������ˮ��D���������ˮ��Ӧ����������D�а���������������������F��NO��������Ӧ�õ�����������G�ж��������ܽ�õ����ᣬ������Cu��Ӧ���ݴ˷������
(1)A��������þ���Ȼ���ټ��������·�Ӧ����MgOHCl��NH3��H2O����Ӧ����ʽΪ��Mg(OH)2+NH4Cl ![]() MgOHCl+NH3��+H2O���ʴ�Ϊ��Mg(OH)2+NH4Cl
MgOHCl+NH3��+H2O���ʴ�Ϊ��Mg(OH)2+NH4Cl ![]() MgOHCl+NH3��+H2O��
MgOHCl+NH3��+H2O��
(2)��ˮ���Ȼ�����Ӧ�����Ȼ�狀�������������Ӧ�����ӷ���ʽΪAl3++3NH3H2O=Al(OH)3��+3NH4+���ʴ�Ϊ��Al3++3NH3H2O=Al(OH)3��+3NH4+��
(3)G�ж���������������������ˮ���ܽ�ᵼ�µ�����ͨ�뵪������ϡ�Ͱ�������ֹ�������ʴ�Ϊ��ϡ�Ͱ�������ֹ������
(4)����ü�ʯ�ҵ�����������a g�������ɵ�ˮ������Ϊag������Mg(OH)2+NH4Cl ![]() MgOHCl+NH3��+H2O�����ɵļ�ʽ�Ȼ�þ������Ϊ
MgOHCl+NH3��+H2O�����ɵļ�ʽ�Ȼ�þ������Ϊ![]() ��76.5g/mol=4.25a g���ʴ�Ϊ��4.25a��
��76.5g/mol=4.25a g���ʴ�Ϊ��4.25a��
(5)��ɫ����ΪCu��Cu2O�����������������������Ϊ���ٵ���Ԫ������������ٵ���Ԫ������Ϊ8g-6.8g=1.2g����CuO����Ԫ������Ϊ8.0g�� ![]() =1.6g��1.2g���ʺ�ɫ����ΪCu��Cu2O������������ʵ����ֱ�Ϊxmol��ymol����x+2y��
=1.6g��1.2g���ʺ�ɫ����ΪCu��Cu2O������������ʵ����ֱ�Ϊxmol��ymol����x+2y��![]() ��64x+144y�����x=0.05��y=0.025����ת�Ƶ���Ϊ0.05mol��2+0.025mol��2=0.15mol���ʴ�Ϊ��Cu��Cu2O��0.15��
��64x+144y�����x=0.05��y=0.025����ת�Ƶ���Ϊ0.05mol��2+0.025mol��2=0.15mol���ʴ�Ϊ��Cu��Cu2O��0.15��
(6)Cu����Ũ���ᷴӦ��Cu2O����ϡ�ᷴӦ�õ�Cu2+����ϡH2SO4��Һ�ܽ⣬��Һ�г�����ɫ��˵����ɫ�����к���Cu2O���ʴ�Ϊ�����Թ��м�������2 mol��L��1H2SO4��Һ����Һ�����ɫ��˵����ɫ�����к���Cu2O��

 ͬ��������ϰϵ�д�
ͬ��������ϰϵ�д� �ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�
�ο�ͨ�γ̱�˼ά����������ѵ��ϵ�д�