��Ŀ����
�����£���20mL0.2mol/LH2A��Һ�еμ�0.2mol/LNaOH��Һ���й��������ʵ����仯����ͼ�����Т����H2A�������HA-�������A2-��������ͼͼʾ�жϣ�����˵��������ǣ� ��
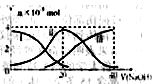
A����V��NaOH��=20mLʱ����Һ������Ũ�ȴ�С��ϵ��c(Na+)>c(HA����>c(H+)> c(A2��)>c(OH��)
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�Ĵ�
C��NaHA��Һ�У�c(OH-)��c(A2�C)��c(H+)��c(H2A)
D����Na2A��Һ����ˮ�Ĺ����У�pH��С

A����V��NaOH��=20mLʱ����Һ������Ũ�ȴ�С��ϵ��c(Na+)>c(HA����>c(H+)> c(A2��)>c(OH��)
B���������Ũ�ȵ�NaOH��Һ��H2A��Һ��Ϻ�����Һ��ˮ�ĵ���̶ȱȴ�ˮ�Ĵ�
C��NaHA��Һ�У�c(OH-)��c(A2�C)��c(H+)��c(H2A)
D����Na2A��Һ����ˮ�Ĺ����У�pH��С
B
���������A����V��NaOH��="20" mLʱ��������ӦΪNaOH+H2A=NaHA+H2O����Һ��ҪΪNaHA����ͼ��֪c��A2-����c��H2A����˵��HA-�������ˮ��̶ȣ���Һ�����ԣ���c��Na+����c��HA-����c��H+����c��A2-����c��OH-������ȷ��B����ͼʾ��ϵ֪��c��A2-����c��H2A����˵���������ˮ��̶ȣ���Һ�����ԣ�ˮ�ĵ����ܵ������ƣ�����C�����ݵ���غ��c��OH-��+2c��A2-��+c��HA-��=c��H+��+c��Na+�������������غ��c��Na+��=c��HA-��+c��H2A��+c��A2-�������Ե�c��OH-��+c��A2-��=c��H+��+c��H2A������ȷ��D��Na2A��Һ��ˮϡ�ͣ��ٽ���ˮ�⣬����Һ������������Ũ�ȼ�С��������Һ��pH��С����ȷ����ѡB��

��ϰ��ϵ�д�
 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
�����Ŀ
 N2H4(g)��
N2H4(g)�� a��c(Na+)= c(H2S)+c(HS?)+2c(S2?)
a��c(Na+)= c(H2S)+c(HS?)+2c(S2?) ��4����ҵ������·ѭ����������������Ĺ�����������ͼ��ʾ��
��4����ҵ������·ѭ����������������Ĺ�����������ͼ��ʾ��

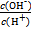 =1��10-12,����˵������ȷ����
=1��10-12,����˵������ȷ���� H++A-
H++A-