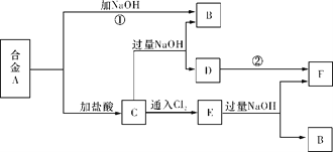
��Ŀ����
����Ŀ��ij�¶�ʱ��VIAԪ�ص�����H2��Ӧ������̬H2X���Ȼ�ѧ����ʽ���£�
![]() O2(g) +H2(g) ��H2O(g)
O2(g) +H2(g) ��H2O(g) ![]() H����242kJ��mol��1
H����242kJ��mol��1
S(g)+ H2(g) ��H2S(g) ![]() H����20kJ��mol��1
H����20kJ��mol��1
Se(g)+H2(g)![]() H2Se(g)
H2Se(g) ![]() H��+81kJ��mol��1
H��+81kJ��mol��1
����˵����ȷ����
A. �ȶ��ԣ�H2O< H2S< H2Se
B. ����������Se��H2��Ӧ����H2Se
C. O2(g)+2H2S(g)��2H2O(g)+2S(g) ![]() H����444 kJ��mol��1
H����444 kJ��mol��1
D. ���ź˵���������ӣ�VIA��Ԫ�ص�����H2�Ļ��Ϸ�ӦԽ������
���𰸡�C
��������
A�Ԫ�صķǽ�����Խǿ����Ӧ���⻯����ȶ���Խǿ��ͬһ���壬���ϵ��£��ǽ���������������ȶ��ԣ�H2O> H2S> H2Se����A�����
B�Se(g)+H2(g)![]() H2Se(g)��Ӧ����H>0��˵���÷�Ӧ�������ȣ�����ʹ�÷�Ӧ����ȵķ����ƶ����������ƶ�������������H2Se����B�����
H2Se(g)��Ӧ����H>0��˵���÷�Ӧ�������ȣ�����ʹ�÷�Ӧ����ȵķ����ƶ����������ƶ�������������H2Se����B�����
C�����֪�Ȼ�ѧ����ʽ���α��Ϊ���������ۣ����ݸ�˹���ɣ���2![]() (��-��)�ɵã�O2(g)+2H2S(g)��2H2O(g)+2S(g)
(��-��)�ɵã�O2(g)+2H2S(g)��2H2O(g)+2S(g) ![]() H����444 kJ��mol��1����C����ȷ��
H����444 kJ��mol��1����C����ȷ��
D����ź˵�����ļ�С����A��Ԫ�صķǽ���������ǿ������ԭ���γɵĹ��ۼ�Խǿ���ų�������Խ�࣬������Խ�ȶ�����A��Ԫ�صĵ�����H2�Ļ��Ϸ�ӦԽ����������D�����
����������������ȷ��ΪC��

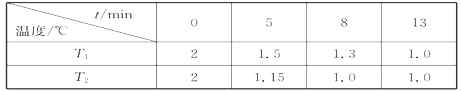
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�