��Ŀ����
��ҵ��ˮ�г�����һ������Cr2O72����CrO42�������ǻ�����༰��̬ϵͳ�����ܴ���������д��������õĴ������������֡�����һ�ַ����ǻ�ԭ���������÷��Ĺ�������Ϊ��
CrO42�� Cr2O72��
Cr2O72��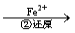 Cr3+
Cr3+ Cr(OH)3��
Cr(OH)3��
���еڢٲ�����ƽ�⣺2CrO42��(��ɫ)+2H�� Cr2O72��(��ɫ)+H2O�������й�˵����ȷ����
Cr2O72��(��ɫ)+H2O�������й�˵����ȷ����
A���ڢٲ���2v(Cr2O72)=v(CrO42��)ʱ���ﵽ��ƽ��״̬
B����������ƽ�⣬��������ϡ�������Һ��ɫ���ɫ���������� CrO42-������
C���ڢڲ��У���ԭ 0.1molCr2O72-��Ҫ45.6gFeSO4
D���ڢ۲�������a����ʹ��NaOH�ȼ�������
 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д���֪��25��ʱ���й�����ĵ���ƽ�ⳣ��������ѡ������ȷ����
���� | H2C2O4 | CH3COOH | HCN | H2CO3 |
���볣��Ki | Ki1=5.9��l0-2 Ki2=6.4��l0-5 | 1.8��l0-5 | 4.9��l0-10 | Ki1=4.3��l0-7 Ki2=5.6��l0-11 |
A�������ʵ���Ũ�ȵ���ҺpH��ϵ��NaHCO3��NaCN��CH3COONa��NaHC2O4
B����ӦNaHC2O4+NaHCO3��Na2C2O4+H2O+CO2���ܷ���
C������������ʵ���Ũ�ȵ���Һ������������NaCN��CH3COONa
D��Na2CO3��Һ��2c(Na+)=c(CO32-)+c(HCO3-)+c( H2CO3)


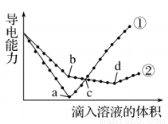
 CO(g)+H2(g)��һ�ɱ��ݻ����ܱ������н��У����������ĸı���䷴Ӧ���ʼ�����Ӱ�����
CO(g)+H2(g)��һ�ɱ��ݻ����ܱ������н��У����������ĸı���䷴Ӧ���ʼ�����Ӱ����� M2+(aq)+2OH-(aq) Ksp=a��c(M2+)="b" mol��L-1ʱ����Һ��pH����
M2+(aq)+2OH-(aq) Ksp=a��c(M2+)="b" mol��L-1ʱ����Һ��pH����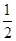 lg(
lg(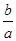 ) B��
) B��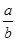 )
) SO3(g) ��H����98.32kJ/mol���������г���2molSO2��1molO2��ַ�Ӧ�����շų�������Ϊ196.64kJ
SO3(g) ��H����98.32kJ/mol���������г���2molSO2��1molO2��ַ�Ӧ�����շų�������Ϊ196.64kJ