��Ŀ����
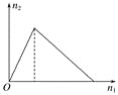
����Ŀ����ҵ�Ϻϳɰ���Ӧ�������仯��ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ��
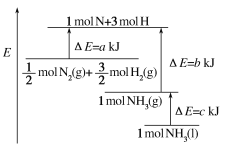
A. N2(g)��3H2(g)=2NH3(l) ��H��2(a��b��c)kJ��mol��1
B. N2(g)��3H2(g)=2NH3(g) ��H��2(b��a)kJ��mol��1
C. ![]() N2(g)��
N2(g)��![]() H2(g)=NH3(l) ��H��(b��c��a)kJ��mol��1
H2(g)=NH3(l) ��H��(b��c��a)kJ��mol��1
D. ![]() N2(g)��
N2(g)��![]() H2(g)=NH3(g) ��H��(a��b)kJ��mol��1
H2(g)=NH3(g) ��H��(a��b)kJ��mol��1
���𰸡�A
��������
���ݷ�Ӧ�ȵ��ڷ�Ӧ����������ȥ���������������㷴Ӧ�Ȳ���д�Ȼ�ѧ����ʽ��ע�ⷴӦ������ʵ�����������ľۼ�״̬��
��ͼ���Կ�����![]() molN2(g)+
molN2(g)+![]() molH2(g)������ΪakJ��1molNH3(g)������ΪbkJ������
molH2(g)������ΪakJ��1molNH3(g)������ΪbkJ������![]() N2(g)+
N2(g)+![]() H2(g)=NH3(g)����H=(a-b)kJ/mol��
H2(g)=NH3(g)����H=(a-b)kJ/mol��
��1mol��NH3(g)ת��Ϊ1mol��NH3(l)�ų�������ΪckJ�������У�![]() N2(g)+
N2(g)+![]() H2(g)=NH3(l)����H=(a-b-c)kJ/mol������N2(g)+3H2(g)=2NH3(1)����H=2(a-b-c)kJmol-1���ʴ�ΪA��
H2(g)=NH3(l)����H=(a-b-c)kJ/mol������N2(g)+3H2(g)=2NH3(1)����H=2(a-b-c)kJmol-1���ʴ�ΪA��

��ϰ��ϵ�д�
 Сѧ��10���ӿ������100��ϵ�д�
Сѧ��10���ӿ������100��ϵ�д�
�����Ŀ