��Ŀ����
�����Ȼ�ѧ����ʽ�У���H����ȷ��ʾ���ʵ�ȼ���ȵ���
| A��C(s) +1/2O2(g) ="=CO(g);" ��H��-110.5 kJ/mol |
| B��CO(g) +1/2O2(g) ==CO2(g); ��H��-283.0 kJ/mol |
| C��H2(g) +1/2O2(g)==H2O(g); ��H��-241.8 kJ/mol |
| D��2C8H18(l) +25O2(g)==16CO2(g)+18H2O(l); ��H��-11036 kJ/mol |
B
����ȼ���ȵ��жϡ�ȼ������ָ��һ�������£�1mol��ȼ����ȫȼ�������ȶ���������ʱ���ų�������������ѡ��B��ȷ��A��Ӧ����CO2��C��Ӧ����Һ̬ˮ��D�в���1mol����ѡB��

��ϰ��ϵ�д�
 ϰ�⾫ѡϵ�д�
ϰ�⾫ѡϵ�д�
�����Ŀ
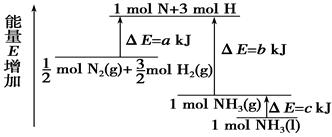
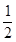 H2(g)+
H2(g)+ 2NH3��g�� +
2NH3��g�� + 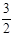 O2��g�� ����H =" a" kJ��mol��1
O2��g�� ����H =" a" kJ��mol��1 2H2O��l�� ��H = ��571��6kJ��mol��1
2H2O��l�� ��H = ��571��6kJ��mol��1