��Ŀ����
��ͼ�ĸ������ʾ�йص�һ�ַ�Ӧ��������ijЩ�����Ѿ���ȥ�������г�����A��C��DΪ��ɫ���壬C��ʹʪ��ĺ�ɫʯ����ֽ������1molX�ֽ�õ�A��B��C��1mol��

��1��д�����и����ʵĻ�ѧʽ��
X�� ��B�� ��F�� ��G�� ��
��2��д�����б仯�ķ�Ӧ����ʽ��
A��D�� ��
C��E�� ��
��3��д�����б仯�����ӷ���ʽ��
G��E��
G��F��
��4��д��ʵ���Һ�ҵ����C�Ļ�ѧ����ʽ��

��1��д�����и����ʵĻ�ѧʽ��
X�� ��B�� ��F�� ��G�� ��
��2��д�����б仯�ķ�Ӧ����ʽ��
A��D�� ��
C��E�� ��
��3��д�����б仯�����ӷ���ʽ��
G��E��
G��F��
��4��д��ʵ���Һ�ҵ����C�Ļ�ѧ����ʽ��
��1��(4��)д�����и����ʵĻ�ѧʽ��
X�� NH4HCO4 ��B�� H2O ��F�� NO2 ��G�� HNO3 ��
��2��(4��)C��E��2CO2 + 2Na2O2 ="=" 2Na2CO3 + O2 ��
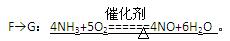
��3��(4��)G��E��3Cu+8HNO3��ϡ��===3Cu(NO3)2+2NO��+4H2O
G��F��Cu+4HNO3��Ũ��=Cu(NO3)2+2NO2��+2H2O
��4��(4��)NH4Cl+Ca��OH)2=====CaCl2+NH3��+2H2O

X�� NH4HCO4 ��B�� H2O ��F�� NO2 ��G�� HNO3 ��
��2��(4��)C��E��2CO2 + 2Na2O2 ="=" 2Na2CO3 + O2 ��
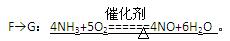
��3��(4��)G��E��3Cu+8HNO3��ϡ��===3Cu(NO3)2+2NO��+4H2O
G��F��Cu+4HNO3��Ũ��=Cu(NO3)2+2NO2��+2H2O
��4��(4��)NH4Cl+Ca��OH)2=====CaCl2+NH3��+2H2O

��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 Ԫ��ԭ�ӵ��������������������������=3/4����C��Eͬ���塣
Ԫ��ԭ�ӵ��������������������������=3/4����C��Eͬ���塣
 1molF���뷴Ӧ��ʱ��ת�Ƶĵ�����ĿΪ ����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ ��������Dͨ��BaCl2��Һ�У�δ�����ǣ����Ҫʹ��Һ����ǣ�������Һ��ͨ�˵����������
1molF���뷴Ӧ��ʱ��ת�Ƶĵ�����ĿΪ ����Ӧ�����������뻹ԭ�������ʵ���֮��Ϊ ��������Dͨ��BaCl2��Һ�У�δ�����ǣ����Ҫʹ��Һ����ǣ�������Һ��ͨ�˵����������  (��2��)��
(��2��)�� ����Ӧ�۵����ӷ���ʽ�� ��2molG��lmolB��������2mol�����ʣ������ʵķ���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ�������ʽΪ ��
����Ӧ�۵����ӷ���ʽ�� ��2molG��lmolB��������2mol�����ʣ������ʵķ���������ԭ�Ӷ��ﵽ8�����ȶ��ṹ�������ʽΪ ��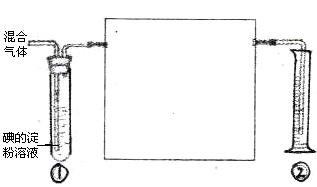


 ��F�����ӷ���ʽ��_____________________________________________
��F�����ӷ���ʽ��_____________________________________________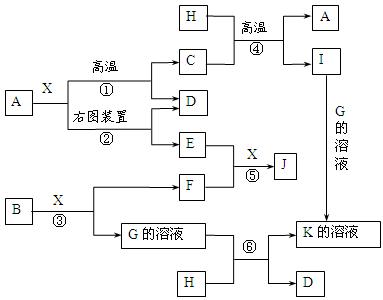
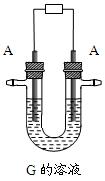
 ��Ҫ����գ�
��Ҫ����գ�