��Ŀ����
����Ŀ����֪CO�ǹ�ҵ����Ҫ��ԭ�ϣ�����ȼ�ϡ�ұ���������ϳ�Һ��ƾ��ȡ�
(1)�о���������ӦCO(g)��H2O(g) ![]() H2(g)��CO2(g) �SH = ?
H2(g)��CO2(g) �SH = ?
ƽ�ⳣ�����¶ȵı仯���±���ʾ��
�¶�/�� | 400 | 500 | 800 |
ƽ�ⳣ��K | 9.94 | 9 | 1 |
��.ͨ��ƽ�ⳣ�����¶ȵı仯������ƶϷ�Ӧ�ȨSH________ 0 ����������� ��
��.����Ӧ��500��ʱ���У�����ʼ��CO��H2O��Ũ�Ⱦ�Ϊ0.020 mol��L��1���ڸ�������CO��ƽ��ת����Ϊ________��
(2)��CO��ȼ�ϵ�ص��CuSO4��Һ��FeCl3��FeCl2���Һ��ʾ��ͼ��ͼ1��ʾ������A��B��D ��Ϊʯī�缫��CΪͭ�缫������һ��ʱ��Ͽ�K����ʱA��B�����ϲ��������������ͬ��
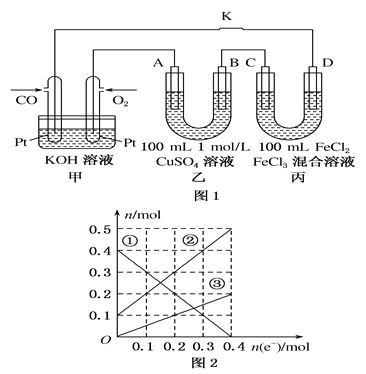
�ټ���ͨ��CO�ĵ缫Ϊ______��������������������������������������õ缫��Ӧ����ʽΪ__________��
��������A�������������ڱ�״���µ����Ϊ2.24L,��ʱҪʹ����CuSO4��Һ�ָ���ԭ����Ũ�ȣ���Ҫ��������ʼ������ʵ�������___________����
A.0.1molCuO B.0.1molCu��OH��2 C.0.1molCu2��OH��2CO3
�۱�װ����Һ�н��������ӵ����ʵ�����ת�Ƶ��ӵ����ʵ����仯��ϵ��ͼ2��ʾ����ɱ�װ��C��D�缫��Ӧʽ��C��____________________________��D��________________________________����װ����Һ�� c��Cl-��= _______________ mol��L ��
���𰸡� �� 75�� �� CO �C 2e- +4OH- =CO32- + 2H2O B Cu �C 2e- = Cu2+ 2Fe3+ +2e- =2Fe2+ 14
��������(1).I. �ɱ������ݿ�֪�������¶ȣ�ƽ�ⳣ��K��С��˵�������¶�ƽ�������ƶ����÷�Ӧ������ӦΪ���ȷ�Ӧ����H��0���ʴ�Ϊ������
��. ����Ӧ��500��ʱ���У���ʼCO��H2O��Ũ�Ⱦ�Ϊ0.02mol/L������ƽ������ʽ���У�
CO(g)��H2O(g) ![]() H2(g)��CO2(g)
H2(g)��CO2(g)
��ʼŨ��(mol/L)�� 0.02 0.02 0 0
ת��Ũ��(mol/L)�� x x x x
ƽ��Ũ��(mol/L)�� 0.02-x 0.02-x x x
��K=![]() =9�����x=0.015mol/L����CO��ƽ��ת����Ϊ��
=9�����x=0.015mol/L����CO��ƽ��ת����Ϊ�� ![]() ��100%=75%���ʴ�Ϊ��75%��
��100%=75%���ʴ�Ϊ��75%��
(2). ��. ���������֪��CO��O2��KOH��Һ����ȼ�ϵ�أ�ͨ��ȼ�ϵĵ缫Ϊ�����������ͨ��CO�ĵ缫Ϊ�������ڸ�����COʧ��������̼������ӣ������ĵ缫��ӦʽΪ��CO �C 2e�� +4OH�� =CO32�� + 2H2O���ʴ�Ϊ��CO �C 2e�� +4OH�� =CO32�� + 2H2O��
��. ��ͼ��֪��A�缫����ȼ�ϵ�ص�������AΪ������A���Ϸ����ĵ缫��ӦʽΪ��4OH���C 4e�� =O2�� + 2H2O��������O2�ڱ�״���µ����Ϊ2.24L����O2�����ʵ���Ϊ0.1mol��ת�Ƶ���0.4mol��BΪ�������Ͽ�K��A��B�����ϲ��������������ͬ����B���Ϸ����ĵ缫��Ӧʽ����Ϊ��Cu2��+2e��= Cu��2H��+2e��=H2����H2�����ʵ���Ϊ0.1mol����Ӧת�Ƶ���0.2mol��������Cuʱת�Ƶĵ���Ϊ0.4mol��0.2mol=0.2mol������Cu2��+2e��= Cu��֪����ʱ����0.1molCu��Ҫʹ����CuSO4��Һ�ָ���ԭ����Ũ�ȣ���ʱ��Ҫ�����������Ӧ����0.1molCu��0.2molH��0.2molO���ʴ�Ϊ��B��
��. ��ͼ1��֪��C���������缫����ΪCu��Cu�ǻ��Ե缫����C�ĵ缫��ӦʽΪ��Cu �C 2e��= Cu2+��D���������缫����Ϊʯī��D�ĵ缫��ӦʽΪ��2Fe3+ +2e- =2Fe2+�����ͼ2��֪������1����Fe3+������2����Fe2+������3����Cu2+�����ǰ��Һ��Fe3+�����ʵ���Ϊ0.4mol��Fe2+�����ʵ���Ϊ0.1mol����n(Cl��)=0.4mol��3+0.1mol��2=1.4mol��c(Cl��)=1.4mol��0.1L=14mol/L���ʴ�Ϊ��Cu �C 2e��= Cu2+��2Fe3+ +2e- =2Fe2+��14��

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д�����Ŀ���״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������ҵ��һ������������ַ�Ӧ�ϳɼ״���
��Ӧ��CO(g)��2H2(g) ![]() CH3OH(g)����H1
CH3OH(g)����H1
��Ӧ��CO2(g)��3H2(g) ![]() CH3OH(g)��H2O(g)����H2
CH3OH(g)��H2O(g)����H2
�±����������Ƿ�Ӧ���ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ��(K��)��
�¶� | 250 �� | 300 �� | 350 �� |
K�� | 2.0 | 0.27 | 0.012 |
��1����һ�������½�2 mol CO��6 mol H2����2 L���ܱ������з�����Ӧ��5 min����c(CO)��0.4 mol��L��1������ɵô˶�ʱ��ķ�Ӧ����(��H2��ʾ)Ϊ________mol��L��1��min��1��
��2���ɱ��������жϦ�H1______(�>����<������)0����ӦCO2(g)��H2(g) ![]() CO(g)��H2O(g)����H3��_________(�æ�H1�ͦ�H2��ʾ)��
CO(g)��H2O(g)����H3��_________(�æ�H1�ͦ�H2��ʾ)��
��3���������ݻ����䣬�����д�ʩ����߷�Ӧ����COת���ʵ���______(�����)��
a������CO��ʹ��ϵ��ѹǿ����
b����CH3OH(g)����ϵ�з���
c������He��ʹ��ϵ��ѹǿ����
d��ʹ�ø�Ч����
��4��д����Ӧ��Ļ�ѧƽ�ⳣ������ʽ��K����__________________�����ֺ��º��ݣ�����Ӧ���ƽ����ϵ�и�����Ũ�Ⱦ�����Ϊԭ����2������ѧƽ��_______(�����������)�ƶ���ƽ�ⳣ��K��__________(������С�����䡱)��
��5���Ƚ������ֺϳɼ״��ķ�����ԭ�������ʽϸߵ���______(���)��





