��Ŀ����
(8��)
I��֪2H2(g)+O2(g)=2H2O(g) ��H=-483��6 kJ��mol
2H2(g)+O2(g)=2H2O(l) ��H=-571��6 kJ��mol
(1)������ȼ���ȡ�H= kJ��mol
(2)ȼ��2gH2����ˮ�������ų�������Ϊ kJ��
���ú��Ϊȼ�Ͽ�ͨ����������;�����������
;��1��ֱ��ȼ��
C(s)+O2(g)=CO2(g) ��H=E1 ��
;��2�����Ƴ�ˮú������ȼ��ˮú��
C(s)+H2O(g)=CO(g)+H2(g) ��H=E2 ��
H2(g)+1��2 O2(g)=H2O(g) ��H=E3 ��
CO(g)+1��2 O2(g)=CO2(g) ��H=E4 ��
��ش�
(1)�����ĸ��Ȼ�ѧ����ʽ���ĸ���Ӧ��H >0? (�����)
(2)��������ú�ֱ�ͨ������������ͬ��;�������Ŀ����õ���������ϵ��ȷ����
(��ѡ����ĸ)��
A��;��1��;��2�� B��;��1��;��2�� C��;��1��;��2����������ͬ
(3)���������غ㶨�ɣ�E1��E2��E3��E4֮��Ĺ�ϵΪ ��
I��֪2H2(g)+O2(g)=2H2O(g) ��H=-483��6 kJ��mol
2H2(g)+O2(g)=2H2O(l) ��H=-571��6 kJ��mol
(1)������ȼ���ȡ�H= kJ��mol
(2)ȼ��2gH2����ˮ�������ų�������Ϊ kJ��
���ú��Ϊȼ�Ͽ�ͨ����������;�����������
;��1��ֱ��ȼ��
C(s)+O2(g)=CO2(g) ��H=E1 ��
;��2�����Ƴ�ˮú������ȼ��ˮú��
C(s)+H2O(g)=CO(g)+H2(g) ��H=E2 ��
H2(g)+1��2 O2(g)=H2O(g) ��H=E3 ��
CO(g)+1��2 O2(g)=CO2(g) ��H=E4 ��
��ش�
(1)�����ĸ��Ȼ�ѧ����ʽ���ĸ���Ӧ��H >0? (�����)
(2)��������ú�ֱ�ͨ������������ͬ��;�������Ŀ����õ���������ϵ��ȷ����
(��ѡ����ĸ)��
A��;��1��;��2�� B��;��1��;��2�� C��;��1��;��2����������ͬ
(3)���������غ㶨�ɣ�E1��E2��E3��E4֮��Ĺ�ϵΪ ��
�� ��1��-285.8 ��2�֣� ��2��241.8 ��2�֣�
�� ��1���� ��1�֣� ��2�� C ��1�֣�
��3�� E1=E2+E3+E4 ��2�֣�
�� ��1���� ��1�֣� ��2�� C ��1�֣�
��3�� E1=E2+E3+E4 ��2�֣�
��

��ϰ��ϵ�д�
 ��ѧ��ʦ����ϵ�д�
��ѧ��ʦ����ϵ�д�
�����Ŀ
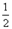 O2(g) ��H1 =" +242" kJ��mol-1
O2(g) ��H1 =" +242" kJ��mol-1 O2(g)="MgO(s) " ��H3 = -602kJ��mol-1
O2(g)="MgO(s) " ��H3 = -602kJ��mol-1
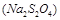 �����ã� ��
�����ã� ��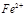 ��Ӧ����������ˮ�����ʣ�����
��Ӧ����������ˮ�����ʣ�����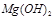 ��Ӧ����Ȼ
��Ӧ����Ȼ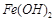 ������ˮ���������������EDTA�ļ��룬�����ܹ���
������ˮ���������������EDTA�ļ��룬�����ܹ��� ��ȥ����ô��ȸߵ�
��ȥ����ô��ȸߵ�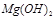 ����ӳ����ܽ�ƽ��ĽǶȼ��Խ��� ��
����ӳ����ܽ�ƽ��ĽǶȼ��Խ��� �� 2NH3(g)�����仯����ͼ���ش��������⣺
2NH3(g)�����仯����ͼ���ش��������⣺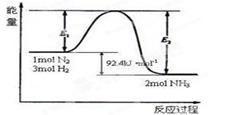
 2NH3(g) ��H��a kJ/mol���Ը��ݱ������м������ݹ���a����ֵ�� ��
2NH3(g) ��H��a kJ/mol���Ը��ݱ������м������ݹ���a����ֵ�� �� 0�֣�����β���к���CO��NO2
0�֣�����β���к���CO��NO2 ���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת���������塣
���ж����壬��������װβ������װ�ã���ʹ�ж��������Ӧת���������塣 ��H�� �� �����ڸ÷�Ӧ���¶Ȳ�ͬ��T2��T1��������������ͬʱ������ͼ����ȷ���� ��
��H�� �� �����ڸ÷�Ӧ���¶Ȳ�ͬ��T2��T1��������������ͬʱ������ͼ����ȷ���� ��  ������ţ���
������ţ���