��Ŀ����
����Ŀ��ij�¶�ʱ��BaSO4��ˮ�еij����ܽ�ƽ��������ͼ��ʾ����ش��������⣺

(1)����˵����ȷ����__________��
A������Na2SO4����ʹ��Һ��a��䵽b��
B��ͨ����������ʹ��Һ��d��䵽c��
C��d����BaSO4��������
D��a���Ӧ��Ksp����c���Ӧ��Ksp
(2)��100 mL 1 mol��L��1H2SO4����100 mL��Ba2��0.137 g����Һ�г�ַ�Ӧ���˳���������Һ�в�����Ba2�������ʵ���Ũ��Ϊ____________��
(3)��������100 mL��ˮ��100 mL 0.01 mol��L��1��H2SO4�ֱ�ϴ�ӣ������������ʧ��BaSO4������֮��Ϊ________��
���𰸡�C 2.02��10��10 mol��L��1 103��1
��������
��1����ͼ�е���ƽ�����ߣ����ϵ�����㶼��ƽ��״̬��b��d����ƽ��״̬�����ѡ������жϣ�
��2����������������뱵���ӵķ�Ӧ�����ʣ������������Ũ�ȣ��ٸ���Ksp���㣻
��3���������Ӷ��ܽ�ƽ���Ӱ�켰Ksp��BaSO4���������ܽ�����ᱵ��������Ȼ��ȷ������ϴ�ӷ�����BaSO4�����������֮�ȡ�
��1��A�����ᱵ��Һ�д������ܽ�ƽ�⣬a����ƽ�������ϣ�����Na2SO4��������c(SO42-)��ƽ�����ƣ�c(Ba2+)Ӧ���ͣ�������Na2SO4����ʹ��Һ��a��䵽b�㣬��A����
B��d��ʱ��Һ�����ͣ������ܼ�ˮ��c(SO42-)��c(Ba2+)������ͨ����������ʹ��Һ��d��䵽c�㣬��B����
C��d���ʾQc��Ksp����Һ�����ͣ������г�����������C��ȷ��
D��Ksp��һ�������¶Ȳ���Ksp���䣬�������ϵ�����һ��Ksp����ȣ���D����
�ʴ�ΪC��
��2����ͼ���֪Ksp(BaSO4)=10-5��10-5=10-10����100mL1molL-1H2SO4��Һ����100mL��Ba2+0.137g����Һ�г�ַ�Ӧ��Ӧ����BaSO4����������ӹ���������n(Ba2+)=0.137g��137g/mol=0.001mol��ʣ�����������ӵ�Ũ��Ϊ��c(SO42-)=��0.1mol��0.001mol����0.2L=0.495mol/L������ʣ��ı�����Ϊ��c(Ba2+)=![]() ��2.02��10-10mol/L��
��2.02��10-10mol/L��
��3����100mL����ˮϴ�ӳ���ʱ���ܽ��BaSO4�����ʵ���Ϊ0.1L��c(Ba2+)=0.1L��![]() mol/L=10-6mol����100mL 0.01molL-1H2SO4��Һϴ��ʱ����������������˳������ܽ⣬���ܽ��BaSO4�����ʵ���Ϊ0.1L��c(Ba2+)=0.1L��
mol/L=10-6mol����100mL 0.01molL-1H2SO4��Һϴ��ʱ����������������˳������ܽ⣬���ܽ��BaSO4�����ʵ���Ϊ0.1L��c(Ba2+)=0.1L��![]() =10-9mol����ͬ�����ʵ�����֮�ȵ������ʵ�����֮�ȿ�֪�����������ʧ��BaSO4������֮��Ϊ10-6mol��10-9mol=1000��1��
=10-9mol����ͬ�����ʵ�����֮�ȵ������ʵ�����֮�ȿ�֪�����������ʧ��BaSO4������֮��Ϊ10-6mol��10-9mol=1000��1��

����Ŀ��ʵ������Ҫ����NaOH��Һ��������Һ��
������100mL 1.0mol��L-1 NaOH��Һ
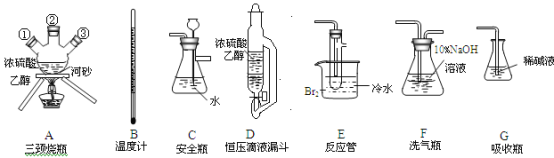
��1����ͼ��ʾ��������E������Ϊ___��������Һ�϶�����Ҫ��������___������ţ����������ӵIJ���������___�����������ƣ���
��2��������NaOH��Һʱ
�ٸ��ݼ�����������ƽ��ȡNaOH������Ϊ___g��
�����в�����������ҺŨ�ȵ�Ӱ����(����ƫ������ƫ��������Ӱ����)��
���� | Ũ��Ӱ�� |
����ƽ��ʹ�����룩����ʱ�����������������λ�÷ŵߵ��� | ___ |
û��ϴ���ձ��Ͳ����� | ___ |
����ʱ�����Ӷ��� | ___ |
����Eδ�����������ˮ | ___ |
������100mL 0.5mol��L-1 ������Һ
����������Ϊ98%���ܶ�Ϊ1.84g��cm-3��Ũ���������Ƹ���Һ����Ũ�������ʵ���Ũ��Ϊ___mol��L-1������Ũ��������Ϊ___mL (����������һλС��)��
����Ŀ�����и��������У���������ͼ��ʾת����ϵ���ǣ���Ӧ������ȥ����ͷ��ʾһ��ת������ ��
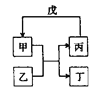
ѡ�� | �� | �� | �� | �� |
A | NH3 | Cl2 | N2 | H2 |
B | C | SiO2 | CO | CuO |
C | Al��OH��3 | NaOH | NaAlO2 | CO2 |
D | Br2 | FeI2 | FeBr3 | Cl2 |
A. AB. BC. CD. D