��Ŀ����
����Ŀ��ij������ˮ�к�����̬�ȣ�ͨ������ʵ��ⶨ��Ũ�ȡ�
��ȡˮ��10��0ml����ƿ�У�����10��0ml��KI��Һ(����)�������ķ�ӦΪ��Cl2+2KI��2KCl+I2������ָʾ��2~3�Ρ�
�ڢ�ȡһ�ζ�������������ˮ������ˮϴ��������0.01mol��L-1Na2S2O3��Һ��ϴ��Ȼ��װ��0.01mol��L-1Na2S2O3��Һ��0�̶����ϣ��ų��¶˼����ڵ����ݣ�����Һ����0�̶Ȼ�0�̶���ijһλ�ã����¶�����
�۽���ƿ���ڵζ����½��еζ��������ķ�ӦΪ��I2+2Na2S2O3=2NaI+ 2Na2S4O6 �Իش������ʴ�
��1������ټ����ָʾ����_______________________________��
��2�������Ӧʹ��________ʽ�ζ��ܡ�
��3���жϴﵽ�ζ��յ��ʵ��������___________________________________��
��4����0.1032mol/L HCl��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ�����������ʵ������Ӱ�����____________
A.��ʽ�ζ���δ�ñ�������Һ��ϴ
B.��ƿδ�ô���Һ��ϴ
C.�ζ�ǰ�ζ��ܼ�������һ���ݣ��ζ���������ʧ��
D.�ζ�ʱ����Һ������ƿ��
��5��̼��H2CO3��K1=4.3��10-7��K2=5.6��10-11,����H2C2O4��K1=5.9��10-2��K2=6.4��10-50.1 mol/L Na2CO3��Һ��pH____________0.1 mol/L Na2C2O4��Һ��pH��(ѡ��������������С��������������)��������Ũ�ȵIJ�����Һ��̼����Һ�������ϣ���Һ�и�������Ũ�ȴ�С��˳����ȷ����_____________��(ѡ����)
A��c(H+)��c(HC2O4-)��c[HCO3-)��c[CO32-) B��c(HCO3-)��c(HC2O4-)��c(C2O42-)��c(CO32-)
C��c(H+)��c(HC2O4-)��c(C2O42-)��c(CO32-) D��c(H2CO3) ��c(HCO3-)��c(HC2O4-)��c(CO32-)
��6����֪�������ܵ���ʵ��ܶȻ�������Ksp��CaF2����1.5��10��10 ��25��ʱ��2.0��10��3mol��L-1�����ˮ��Һ�У�������ҺpH����������仯�����õ�c��HF����c��F-������ҺpH�ı仯��ϵ������ͼ��ʾ�������������Ϣ�ش��������⣺
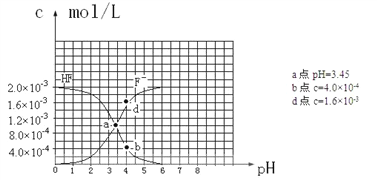
��25��ʱ��HF����ƽ�ⳣ������ֵKa��_______________________________��
��4.0��10��3 mol��L-1HF��Һ��4.0��10��4 mol��L-1 CaCl2��Һ�������ϣ����ڻ��ҺpHΪ4.0�����Ե��ڻ��Һ����ı仯����ͨ����ʽ����˵���Ƿ��г���������_______________________________________
���𰸡� ������Һ �� �������һ�α�Һ����Һ����ɫ�����ɫ�Ұ�����ڲ��ָ� B ���� AC 10-3.45����3.5��10-4�� ��pH=4.0ʱ����Һ����c(F-)=1.6��10-3mol/L����Һ��c(Ca2+)=2.0��l0-4 mol/L��c( Ca2+)��c2(F-)= 5.l��10-10> Ksp( CaF2)���г�������
��������(1)������з�����ӦΪCl2+2KI��2KCl+I2����˿��õ�����Һ��ָʾ����
(2)����Na2S2O3��Һ�ʼ��ԣ�����Ӧ�ü�ʽ�ζ��ܡ�
(3)�ζ�ʱ�����ķ�ӦΪI2+2Na2S2O3=2NaI+ 2Na2S4O6���õ�����Һ��ָʾ������ζ��յ��ʵ�������ǵ������һ�α�Һ����Һ����ɫ����ɫ�����ɫ���ڰ�����ڲ��ָ���
(4)A����ʽ�ζ���δ�ñ�������Һ��ϴ��ʹ����ϡ�ͣ�������Ҫ���������ʹʵ����ƫ�ߣ�B����ƿ������ϴ�������ʹ�ⶨ���ƫ�ߣ�������ƿδ�ô���Һ��ϴ����ʵ������Ӱ�죻C�����ڵζ���������ʧ�����µζ�����Һ���½���ʹ�����������ʵ����ƫ�ߣ�D���ζ�ʱ��С�Ľ���Һ������ƿ�⣬���±�Һ�������ʹʵ����ƫ�ߡ�������ȷ��ΪB��
(5)��֪����ĵ��볣��K1����̼���K1������ĵ��볣��K2Ҳ����̼���K2���������ࡰԽ��Խˮ������ԭ����ͬŨ�ȵ�Na2CO3��Һˮ��̶ȴ���Na2C2O4��Һ�ģ����Na2CO3��Һ��pH����ͬŨ��Na2C2O4��Һ��pH�����ڶ�Ԫ�����Ƿֲ�����ģ����������K1��K2�Ĺ�ϵ����ȷ��A��C����ȷ��B����ȷ��ϵʽΪc(HC2O4-)�� c(C2O42-)�� c(HCO3-)�� c(CO32-)��D����ȷ��ϵʽΪc(H2CO3) ��c(HC2O4-)��c(HCO3-)��c(CO32-)��
(6) ��25��ʱ��HF����ƽ�ⳣ��Ka=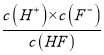 �����Ե�c(F-)=c(HF)ʱ��Ka=c(H+)����ͼ���֪�����еĽ��㴦��Ϊc(F-)=c(HF)������Ӧ��pH=3.45��ΪKa�ĸ���������Ka��10-3.45����3.5��10-4������4.0��10��3 mol��L-1HF��Һ��4.0��10��4 mol��L-1 CaCl2��Һ�������ϵ�˲����Һ��c(HF)=2.0��10-3mol/L����˿���ʹ��ͼ���е����ݽ�����ؼ�������ͼ���֪����pH=4.0ʱ����Һ�е�c(F-)=1.6��10-3mol/L������Ϻ���Һ��c(Ca2+)=2.0��l0-4mol/L������c(Ca2+)��c2(F-)= 2.0��l0-4��(1.6��10-3)2 = 5.l2��10-10 > Ksp(CaF2) ��1.5��10��10�����Ի��г���������
�����Ե�c(F-)=c(HF)ʱ��Ka=c(H+)����ͼ���֪�����еĽ��㴦��Ϊc(F-)=c(HF)������Ӧ��pH=3.45��ΪKa�ĸ���������Ka��10-3.45����3.5��10-4������4.0��10��3 mol��L-1HF��Һ��4.0��10��4 mol��L-1 CaCl2��Һ�������ϵ�˲����Һ��c(HF)=2.0��10-3mol/L����˿���ʹ��ͼ���е����ݽ�����ؼ�������ͼ���֪����pH=4.0ʱ����Һ�е�c(F-)=1.6��10-3mol/L������Ϻ���Һ��c(Ca2+)=2.0��l0-4mol/L������c(Ca2+)��c2(F-)= 2.0��l0-4��(1.6��10-3)2 = 5.l2��10-10 > Ksp(CaF2) ��1.5��10��10�����Ի��г���������

����Ŀ��ij����С�����������ʵ��ⶨCu��Ag�Ͻ���Ũ����ķ�Ӧ����ʵ��װ����ͼ��

��ش��������⣺
��1��F������������_______________��������____________________________��
��2��ʵ������NH4Cl����ͱ���NaNO2��Һ�ڼ����������Ʊ�N2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��װ��A��ʢ�ŵ������Ը��������Һ��������___________________________________________��
��3������ʵ�顣���װ�������Ժ�,��װ����������Ӧ���Լ�,Ȼ��__________����������������ٽ�Bװ�÷�Һ©���е�Ũ���Ỻ���μӵ�������ƿ�С�
��4���ⶨ��������ʵ�������Ӧ����������װ��D������500mL��Һ��ÿ��ȡ��25.00mL��Һ������2��ָʾ������0.05 mol��L-1��NaOH��Һ�ζ������εζ������������±���
�ζ�ǰ���/mL | �ζ������/mL | |
��һ�� | 0.33 | 20.32 |
�ʶ��� | 1.25 | 23.26 |
��=.�� | 1.47 | 21.48 |
װ��D�����ɵ�����Ϊ________mol����Cu-Ag�Ͻ���Ũ���ᷴӦ���������ɵ�NO2�ڱ�״���µ����Ϊ__________mL��
��5���ⶨNO��������ڲⶨNO�����ʱ����E����������ˮ��Һ��ȸ���ܵ�Һ��ߣ�ֱ�Ӷ�����ʹ�ⶨ���������_________���ƫ��ƫС��������ʱӦ������Ͳ��λ��_______������ơ������ơ������Ա�֤����Ͳ�е�Һ���������е�Һ���ƽ��

