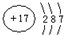��Ŀ����
��14�֣�X ��Y ��Z ��W ��R �����ֶ�����Ԫ�أ���ԭ��������������X �����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Y Ԫ��ԭ�ӵ�������������������������� 3����Z ��W ��R ����ͬһ���ڣ�R �� Y ����ͬһ�壬Z ��W ԭ�ӵĺ��������֮����Y ��R ԭ�ӵĺ��������֮����ȡ�����д���пհף�
��1������ W ��ԭ�ӽṹʾ��ͼ�� ��
��2��Z ��W ����������ˮ����ļ��Խ�ǿ���ǣ� ��д��ѧʽ��������֮������Һ�з�Ӧ�����ӷ���ʽ�ǣ� ��
��3��Z2Y2 �������ӵĻ�ѧʽ�� ��
��4����ͬ����Ԫ���У�R Ԫ�ؼ���������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���ǣ� ���� RԪ��ͬ�����ҷǽ�������ǿ��Ԫ���ǣ� ��
��5��W�ĵ�����ʯī�Ͷ������ѣ�TiO2����������ϣ������·�Ӧ�õ��Ļ������������Ԫ����ɣ��Ҷ��������մɲ��ϣ��䷴Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��ÿ��2�֣���14�֣���1���ԡ�
��2��NaOH �� Al(OH)3�� OH�� ��AlO2�� �� 2H2O ��
��3��O22���� ��4�� P��S��Cl �� �Ȼ�Cl ��
��5��4Al �� 3C �� 3TiO2 2Al2O3 ��3TiC
����:

 ȫ�ų��100��ϵ�д�
ȫ�ų��100��ϵ�д� Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д�| 1 | ��X��Y�õ������ӣ�����������Һ�У�Y����ʴ |
| 2 | ��X��W�ֱ�Ͷ���Ũ�ȵ�ϡ�����ж��������������W��X��Ӧ���� |
| 3 | �ö��Ե缫��⺬�����ʵ���Ũ�ȵ�Y2+��Z2+�����Һ����������������������Z |
| A��Z2+�������Կ�����ǿ |
| B��W�Ľ������ǿ��Y |
| C��Z����CuSO4��Һ��һ����Cu���� |
| D����X��Z��ϡ���ṹ�ɵ�ԭ��أ�X������ |
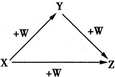 X��Y��Z��W����ͼ��ʾ��ת����ϵ����x��Y�����ǣ�������
X��Y��Z��W����ͼ��ʾ��ת����ϵ����x��Y�����ǣ�������