��Ŀ����
��1������ͼ��Ԫ�����ڱ�p���л�������Ԫ����ǽ���Ԫ�صķֽ��ߣ�
��2������NaH�Ĵ��ڣ���������ɽ���Ԫ�ط��ڢ�A�壬��ô�������������������۵ľ���ֵ��ȣ��ֿɽ���Ԫ�ط������ڱ��е�
��3��ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ����
��4��X��Y��Z��W��Ԫ�����ڱ�ǰ�������е����ֳ���Ԫ�أ��������Ϣ�����
��Yλ��Ԫ�����ڱ�λ��
��XY2��һ�ֳ��õ��ܼ�����д�����ĵ���ʽ
 ��XY2�ķ����д���
��XY2�ķ����д���
��Y�������������仯ѧʽΪYO2��YO3������YO3��Yԭ�Ӳ���
��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��

��2������NaH�Ĵ��ڣ���������ɽ���Ԫ�ط��ڢ�A�壬��ô�������������������۵ľ���ֵ��ȣ��ֿɽ���Ԫ�ط������ڱ��е�
��A
��A
�壮��3��ǰ������Ԫ���У���̬ԭ����δ�ɶԵ���������������������ͬ��Ԫ����
5
5
�֣���4��X��Y��Z��W��Ԫ�����ڱ�ǰ�������е����ֳ���Ԫ�أ��������Ϣ�����
| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | M������2�ԳɶԵ��� |
| Z | Z��Yͬ���ڣ�Z�ĵ縺�Դ���Y |
| W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
��3���ڡ��ڢ�A��
��3���ڡ��ڢ�A��
��Y��Z������������Ӧ��ˮ��������Խ�ǿ����HClO4
HClO4
��д��ѧʽ������XY2��һ�ֳ��õ��ܼ�����д�����ĵ���ʽ


2
2
���Ҽ�����H-Y��H-Z���ֹ��ۼ��У����ļ��Խ�ǿ����H-Z
H-Z
����Y�������������仯ѧʽΪYO2��YO3������YO3��Yԭ�Ӳ���
sp2
sp2
�ӻ���YO2���ӵĿռ乹��V��
V��
����W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��
[Ar]3d104s1
[Ar]3d104s1
�����к�W2+��������ѧʽΪ[W��H2O��4]SO4?H2O����������к��еĻ�ѧ��������λ�������ۼ������Ӽ�
��λ�������ۼ������Ӽ�
����������������H2O
H2O
����������1��p���н���Ԫ����ǽ���Ԫ�صķֽ���λ��B��Al��Si��Ge��As��Sb��Te��Po֮�䣻
��2�����ݵڢ�A��Ԫ�ص��������������۵ľ���ֵ����жϣ�
��3����һ�����У���һ��δ�ɶԵ��ӵ�����ԭ�ӣ�������Ų�Ϊ1s1���ڶ������У�δ�ɶԵ�����������������C��1s22s22p2 �� O��1s22s22p4�����������У�δ�ɶԵ�������������P��1s22s22p63s23p3������������δ�ɶԵ������ĸ�����Fe��1s22s22p63s23p64s23d6��
��4���ɱ��п�֪��X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ�����Ϊ2����XΪC��
Y��M������2�ԳɶԵ��ӣ���֪YΪS��
Z��Sͬ���ڣ�Z�ĵ縺�Դ���S�Ľ���Cl����ZΪCl��
W��һ�ֺ��ص�������Ϊ63��������Ϊ34����������Ϊ29��29��Ԫ��Ϊͭ����WΪCu��
��϶�Ӧ���ʡ�������������Լ���ĿҪ������⣮
��2�����ݵڢ�A��Ԫ�ص��������������۵ľ���ֵ����жϣ�
��3����һ�����У���һ��δ�ɶԵ��ӵ�����ԭ�ӣ�������Ų�Ϊ1s1���ڶ������У�δ�ɶԵ�����������������C��1s22s22p2 �� O��1s22s22p4�����������У�δ�ɶԵ�������������P��1s22s22p63s23p3������������δ�ɶԵ������ĸ�����Fe��1s22s22p63s23p64s23d6��
��4���ɱ��п�֪��X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ�����Ϊ2����XΪC��
Y��M������2�ԳɶԵ��ӣ���֪YΪS��
Z��Sͬ���ڣ�Z�ĵ縺�Դ���S�Ľ���Cl����ZΪCl��
W��һ�ֺ��ص�������Ϊ63��������Ϊ34����������Ϊ29��29��Ԫ��Ϊͭ����WΪCu��
��϶�Ӧ���ʡ�������������Լ���ĿҪ������⣮
����⣺��1��p���н���Ԫ����ǽ���Ԫ�صķֽ���λ��B��Al��Si��Ge��As��Sb��Te��Po֮�䣬�ɱ�ʾΪ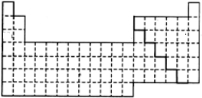 ��
��
�ʴ�Ϊ�� ��
��
��2���ڢ�A��Ԫ�ص��������������۵ľ���ֵ��ȣ�
�ʴ�Ϊ����A��
��3����һ�����У���һ��δ�ɶԵ��ӵ�����ԭ�ӣ�������Ų�Ϊ1s1���ڶ������У�δ�ɶԵ�����������������C��1s22s22p2 �� O��1s22s22p4�����������У�δ�ɶԵ�������������P��1s22s22p63s23p3������������δ�ɶԵ������ĸ�����Fe��1s22s22p63s23p64s23d6����5�֣�
�ʴ�Ϊ��5��
��4���ɱ��п�֪��X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ�����Ϊ2����XΪC��
Y��M������2�ԳɶԵ��ӣ���֪YΪS��
Z��Sͬ���ڣ�Z�ĵ縺�Դ���S�Ľ���Cl����ZΪCl��
W��һ�ֺ��ص�������Ϊ63��������Ϊ34����������Ϊ29��29��Ԫ��Ϊͭ����WΪCu��
��YΪSԪ�أ�ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ6����λ�����ڱ���3���ڵڢ�A�壬ZΪCl���ǽ����Խ�ǿ����Ӧ����ۺ�����ΪHClO4��������ǿ��
�ʴ�Ϊ����3���ڡ��ڢ�A�壻HClO4��
��XY2ΪCS2������ʽΪ ���ṹʽΪS=C=S������2���Ҽ�������Cl�ĵ縺�Ա�Sǿ������H-Y��H-Z���ֹ��ۼ��У����ļ��Խ�ǿ����H-Z��
���ṹʽΪS=C=S������2���Ҽ�������Cl�ĵ縺�Ա�Sǿ������H-Y��H-Z���ֹ��ۼ��У����ļ��Խ�ǿ����H-Z��
�ʴ�Ϊ�� ��2��H-Z��
��2��H-Z��
��S�������������仯ѧʽΪSO2��SO3������SO3��S�γ�3�Ҽ���û�йµ��Ӷԣ�ԭ�Ӳ���sp2�ӻ���SO2��S�γ�2�Ҽ�����1�ŵ��Ӷԣ�ΪV�Σ�
�ʴ�Ϊ��sp2��V�Σ�
��WΪCu��29��Ԫ�أ�������29�����ӣ���̬ԭ�Ӻ�������Ų�ʽ��[Ar]3d104s1���û������к�����λ�������ۼ������Ӽ�������ΪH2O��
�ʴ�Ϊ��[Ar]3d104s1����λ�������ۼ������Ӽ��� H2O��
 ��
���ʴ�Ϊ��
 ��
����2���ڢ�A��Ԫ�ص��������������۵ľ���ֵ��ȣ�
�ʴ�Ϊ����A��
��3����һ�����У���һ��δ�ɶԵ��ӵ�����ԭ�ӣ�������Ų�Ϊ1s1���ڶ������У�δ�ɶԵ�����������������C��1s22s22p2 �� O��1s22s22p4�����������У�δ�ɶԵ�������������P��1s22s22p63s23p3������������δ�ɶԵ������ĸ�����Fe��1s22s22p63s23p64s23d6����5�֣�
�ʴ�Ϊ��5��
��4���ɱ��п�֪��X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������ȣ�����Ϊ2����XΪC��
Y��M������2�ԳɶԵ��ӣ���֪YΪS��
Z��Sͬ���ڣ�Z�ĵ縺�Դ���S�Ľ���Cl����ZΪCl��
W��һ�ֺ��ص�������Ϊ63��������Ϊ34����������Ϊ29��29��Ԫ��Ϊͭ����WΪCu��
��YΪSԪ�أ�ԭ�Ӻ�����3�����Ӳ㣬����������Ϊ6����λ�����ڱ���3���ڵڢ�A�壬ZΪCl���ǽ����Խ�ǿ����Ӧ����ۺ�����ΪHClO4��������ǿ��
�ʴ�Ϊ����3���ڡ��ڢ�A�壻HClO4��
��XY2ΪCS2������ʽΪ
 ���ṹʽΪS=C=S������2���Ҽ�������Cl�ĵ縺�Ա�Sǿ������H-Y��H-Z���ֹ��ۼ��У����ļ��Խ�ǿ����H-Z��
���ṹʽΪS=C=S������2���Ҽ�������Cl�ĵ縺�Ա�Sǿ������H-Y��H-Z���ֹ��ۼ��У����ļ��Խ�ǿ����H-Z���ʴ�Ϊ��
 ��2��H-Z��
��2��H-Z����S�������������仯ѧʽΪSO2��SO3������SO3��S�γ�3�Ҽ���û�йµ��Ӷԣ�ԭ�Ӳ���sp2�ӻ���SO2��S�γ�2�Ҽ�����1�ŵ��Ӷԣ�ΪV�Σ�
�ʴ�Ϊ��sp2��V�Σ�
��WΪCu��29��Ԫ�أ�������29�����ӣ���̬ԭ�Ӻ�������Ų�ʽ��[Ar]3d104s1���û������к�����λ�������ۼ������Ӽ�������ΪH2O��
�ʴ�Ϊ��[Ar]3d104s1����λ�������ۼ������Ӽ��� H2O��
�����������ۺϿ������ʵĽṹ��λ�ú����ʣ�Ϊ�߿��������ͣ�������ѧ���ķ����������ۺ����û�ѧ֪ʶ�������Ŀ��飬��ȷ�ƶ�Ԫ���ǽⱾ��ؼ�����Ŀ�Ѷ��еȣ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��2013?����һģ������ѧһ���ʽṹ�����ʡ�
��2013?����һģ������ѧһ���ʽṹ�����ʡ�

 ��1���ڵ�3�����У��û��������������ǿ��Ԫ�ص�Ԫ�ط���Ϊ
��1���ڵ�3�����У��û��������������ǿ��Ԫ�ص�Ԫ�ط���Ϊ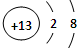
 C��g����������������ʱ���ı�����һ��������������C�����ʣ���ӿ족�����������䡱����?a������
C��g����������������ʱ���ı�����һ��������������C�����ʣ���ӿ족�����������䡱����?a������ ��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���
��2012?�人ģ�⣩[��ѧһѡ�� 3�����ʽṹ������]��U��V��W��X��Y��Z ����ǰ������Ԫ�أ�ԭ���������������������Ϣ���±���