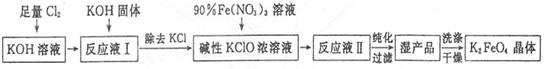
��Ŀ����
��ͼ��ij��ҵ��Ƶ����ᡪ�ʡ�ˮ����������ˮ����ˮ���á��Ρ��ȡ���������������̬��ҵ������ͼ��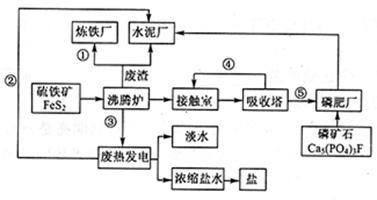
����������ҵ���̻ش��������⣺
��1�������̢١��ڡ��ۡ��ܡ���Ϊ���������ʵ����ͣ���ֱ�д�����͵���Ҫ���ʵĻ�ѧʽ��������ʽ���� ���� ���� ���� ���� ��
��2������¯������Ӧ�Ļ�ѧ����ʽ�� ���ʳ�����Ҫ��Ʒ���ո�(�������ƺ������)��д������ʯ�����ᷴӦ���ոƵû�ѧ����ʽ ��
��3����1��������(FeS2����������Ϊ36��)�Ӵ��������ᣬ������IJ���Ϊ65�������������������������Ϊ98�������� �֡�
��4���ȵ糧����ȴˮ�� ����������Ũ����ˮ����ȡ���������ȡ�������� ��д��һ�ּ��ɣ���
��5�������ִ���������������������¯�������������������������ܵ��������롣
�� ��д�����㼴�ɣ���
��1����Fe2O3 �ڵ��� ������ ��SO2 ��ŨH2SO4 ����1�֣�
��2��4FeS2+11O2 2Fe2O3+8SO2��2�֣�2Ca5(PO4)3F+7H2SO4��3Ca(H2PO4)2+7CaSO4+2HF��2�֣�
2Fe2O3+8SO2��2�֣�2Ca5(PO4)3F+7H2SO4��3Ca(H2PO4)2+7CaSO4+2HF��2�֣�
��3��0��39��2�֣� ��4����ˮ þ���� ��2�֣�
��5����������Ҫ�Ǹ�¯ú���������������Ϊȼ�ϡ� ��2�֣�
��������Ҫ�ɷ��ǹ���Ƶȣ���������ˮ������ԭ�ϡ�
��������Ҫ�ɷ��ǹ���Ƶȣ��������������ʵ�����ԭ�ϡ�
���������������1������ұ��������ԭ����Fe2O3�������ȵ糧�������ṩ������Ϊ���ܣ��۷���¯��FeS2��������Ӧ�ų��������ȣ���������ʱ�Ӵ����ж������������������Ṥҵ�����ɵ����ᣬ���������ʣ�
��2������¯��FeS2��������Ӧ����Fe2O3��SO2���䷴Ӧ����ʽΪ��4FeS2+11O2 2Fe2O3+8SO2������������ʯ��Ӧ�����������ơ�����ƣ�����ԭ���غ��֪�����з��������ɣ���˷�Ӧ�Ļ�ѧ����ʽΪ2Ca5(PO4)3F+7H2SO4��3Ca(H2PO4)2+7CaSO4+2HF��
2Fe2O3+8SO2������������ʯ��Ӧ�����������ơ�����ƣ�����ԭ���غ��֪�����з��������ɣ���˷�Ӧ�Ļ�ѧ����ʽΪ2Ca5(PO4)3F+7H2SO4��3Ca(H2PO4)2+7CaSO4+2HF��
��3������ԭ���غ��֪
FeS2��������������������2H2SO4
120t 2��98t
1t��36%��65% m��98%
��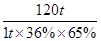 ��
��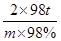
���m��0��39t
��4���غ������зḻ�ĺ�ˮ��Դ����ˮ�к��зḻ��MgԪ�ء���Ԫ�صȿ�����ȡMg���壻
��5�����ݹ����ķ����������ijɷֿ�֪����������Ҫ�Ǹ�¯ú���������������Ϊ�ȷ�¯������¯��¯��ȼ�ϣ���������Ҫ�ɷ��ǹ���Ƶȣ���������ˮ������ԭ�ϣ��ʴ�Ϊ����������Ҫ�Ǹ�¯ú���������������Ϊ�ȷ�¯������¯��¯��ȼ�ϣ���������Ҫ�ɷ��ǹ���Ƶȣ���������ˮ������ԭ�ϣ�
���㣺���鳣��������ת����ʽ�����������Ⱦ�����������ʵķ��롢�ᴿ�Ļ�������ѡ����Ӧ��

 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д� ��˼ά������ҵϵ�д�
��˼ά������ҵϵ�д���ˮ��Դ�ḻ����ˮ���������ѳ�Ϊ��ѧ�ҵ���Ҫ�о�������ʵ�к�ˮ��������Ӧ����ˮ�����з�������ȷ���� ( )
| A������̫���ܣ�����ˮ���� |
| B������������ʹ��ˮ���ηݳ��������� |
| C��ֱͨ���磬���õ�����ʹ��ˮ���� |
| D��ͨ�����ӽ���Ĥ����ȥ�������η�ʹ��ˮ���� |
�����������ڸ��ϲ��ϵ���
| A����ͨ���� | B����ͨ�� | C�������� | D��þ���Ͻ� |
����ѧѡ��-��ѧ�뼼����(15��)
��ʯ�����ȼҵ�е�һ�ַ���������������±���ʾ��
�õ�ʯ����������ˮCaCl2��ij��������������¹������̣�
��֪�Ȼ��ƾ���Ļ�ѧʽ�ǣ�CaCl2��6H2O��H2S��һ���������壬�Ҿ��л�ԭ�ԡ�
�ŷ�Ӧ���м������Ӧѡ��___________________��
����ɫ����Ӧ���������X��_______________���豸A��������______________���豸B������Ϊ________________���豸C��������____________________��
��Ϊ�����㻷��Ҫ���轫����H2Sͨ�����ճأ��������������ʺ���Ϊ���ռ�����____________ _����Ӧ�Ļ�ѧ����ʽΪ_________________��
| A��ˮ | B��Ũ���� | C��ʯ���� | D������ |
���ȼҵ���ӷ���ʽ_____________________��
ij������CaSO4��NH3��H2O��CO2�Ʊ�(NH4)2SO4���乤���������£�
�����ƶϲ��������� (����)��
| A������1 mol(NH4)2SO4��������2 mol NH3 |
| B��CO2�ɱ�ѭ��ʹ�� |
| C��������ͨCO2������(NH4)2SO4������ |
| D��ֱ��������Һ���ɵõ�������(NH4)2SO4 |
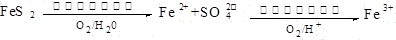
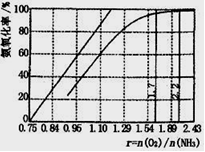

 Fe3++3H2O��ƽ�ⳣ��K= ��
Fe3++3H2O��ƽ�ⳣ��K= ��
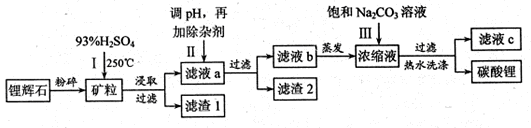
 Li2SO4+Al2O3·4SiO2?H2O
Li2SO4+Al2O3·4SiO2?H2O