��Ŀ����
�������(K2Fe04)��һ�ּ�������������������һ������Ͷ��ˮ���������������������£�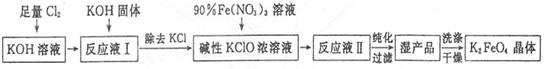
��֪��2Fe(NO3)3+3KClO+10KOH=2K2FeO4+6KNO3+3KCl+5H2O
�ش�����������
��1����Cl2ͨ��KOH��Һ�з�����Ӧ�����ӷ���ʽ��____________��
��2��д����ҵ����ȡCl2�Ļ�ѧ����ʽ____________��
��3���ڡ���ӦҺI���м���KOH�����Ŀ����____________��
��4��K2FeO4����Ϊ���Ͷ��ˮ��������ԭ����____________��
��5������KOH��Һʱ����61.6g KOH�����ܽ���100 mLˮ�У�������Һ���ܶ�Ϊ1.47 g ? mL-1�������Һ�����ʵ���Ũ��Ϊ____________��
��6���ӡ���ӦҺII���з����K2Fe04����Ʒ��___________ (д�� ѧ ʽ����
��7���ù���ÿ�õ�1.98kgK2FeO4������������Cl2�����ʵ���Ϊ______mol��
��1��Cl2+2OH- = Cl-+ ClO-+ H2O��2�֣�
��2��2NaCl + 2H2O 2NaOH + H2�� + Cl2����2�֣�
2NaOH + H2�� + Cl2����2�֣�
��3���롰��ӦҺ���й�����Cl2������Ӧ����KClO��2�֣�
��4��K2FeO4����ǿ�����ԣ���ɱ����������ԭ����FeԪ��Ϊ+3�ۣ���ˮ���γ�Fe(OH)3���壬������ˮ���������γɳ�������2�֣�
��5��10 mol��L-1��3�֣�
��6��KNO3 KCl��2�֣�
��7��15 ��2�֣�
�������������ע��տ�ʼͨ��������Cl2,�����Cl2�����K2FeO4�Ʊ�ʱ�ķ�Ӧ����K2FeO4�Ʊ����̿�֪����ƷΪKNO3��KCl�� (5)�и������ʵ���Ũ�ȶ�����㣬n(KOH)=1.1mol,V=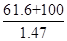 =109.9ml=0.11L�ɵ�C(KOH)= 10 mol��L-1����7������������ԭ�����غ㣬2Fe(NO3)3+3KClO+10KOH=2K2FeO4+6KNO3+3KCl+5H2O
=109.9ml=0.11L�ɵ�C(KOH)= 10 mol��L-1����7������������ԭ�����غ㣬2Fe(NO3)3+3KClO+10KOH=2K2FeO4+6KNO3+3KCl+5H2O
Cl2+2KOH=KCl+KClO+H2O, 2K2Fe04����3Cl2,
n(K2FeO4)=10mol,n(Cl2)=15mol
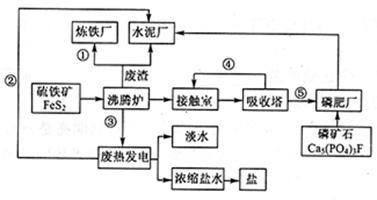
���㣺���⻯���������̿����˷���ʽ���������ʣ�������ԭ������

 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�TiO2�׳��Ѱۣ���;�㷺�������㷺�����������л����Ϊ�¹�ҵ����ҵ������TiO2�Ʊ������ѵ��������¡�
��֪��I��
Ti(s)��O2(g)=TiO2(s) ��H=��946 kJ��mol��1
2C(s)��O2(g)=2CO(g) ��H=��221 kJ��mol��1
Ti(s)��2Cl2(g)=TiCl4(g) ��H=��815 kJ��mol��1
II��ij�¶��¸����ʵķе����£�
| ���� | TiCl4 | FeCl3 | SiCl4 | AlCl3 |
| �е�/�� | 136 | 310 | 56.5 | 180 |
�ش��������⣺
��1�����Ȼ������з�����Ӧ��TiO2(s)+2Cl2(g)+2C(s)
 TiCl4(g)+2CO (g) ���Լ����䷴Ӧ�ġ�H= kJ?mol��1����Ӧ��ƽ�ⳣ������ʽK= ������ͼ������TiCl4�ﵽƽ���ٷֺ������¶ȵı仯����ͼ��
TiCl4(g)+2CO (g) ���Լ����䷴Ӧ�ġ�H= kJ?mol��1����Ӧ��ƽ�ⳣ������ʽK= ������ͼ������TiCl4�ﵽƽ���ٷֺ������¶ȵı仯����ͼ��
��2���Ȼ������д���ĸ�������FeCl3��SiCl4��AlCl3������ ������ýϴ�����TiCl4��
��3��TiO2��Cl2��Ӧ��TiO2��s��+2Cl2��g��
 TiCl4��l��+O2��g����H=+151kJ?mol��1���÷�Ӧ�ڸ��������µ����Է�����������̼��Ӧ��˳�����У��Խ�������ԭ��
TiCl4��l��+O2��g����H=+151kJ?mol��1���÷�Ӧ�ڸ��������µ����Է�����������̼��Ӧ��˳�����У��Խ�������ԭ�� (4)��ԭ����Ҫ�ڶ�������������н��е�������_______________________��
��5����ȡTi���¹������ö�����������������ʯīΪ��������CaCl2����������ʣ�������״̬���ܴ���O2�����������õ�Ti���ù��վ��в������ɱ��ͣ�����Ⱦ���ŵ㣬д������Ʊ�������ʱ�����ĵ缫��Ӧʽ�� ��
���й��ڹ�ҵ������˵���У��������(����)��
| A���ȼҵ�е������ӽ���Ĥ��ֹ������ͨ�� |
| B��������ͨ��������Ҫԭ����ʯ��ʯ��ʯӢ�ʹ��� |
| C����ҵ�Ͻ���ͭ���о�����Ӧ����ͭ�����ڵ�Դ������ |
| D����ҵ�ϣ��ý�̿�ڵ�¯�л�ԭ��������õ����������ʵĴֹ� |
���й��ڻ�������ԭ���ļ��������У�������Ŀǰ��ҵ����ʵ���������(����)
| A��ʯ�������Ļ���ʯ��ҵ�в��ø���ķ�����ʯ�ͷֳɲ�ͬ�е㷶Χ�IJ��� |
| B����������������ڹ��������������Ȼ��⣬����ˮ������������ |
| C�����������ڽӴ��ұ��������������������������ڱ�ˮ�����Ƴ�Ũ���� |
| D���ϳɰ���ҵ�У����ڰ���Һ����N2��H2ѭ��ʹ�ã�����������˵���IJ��ʺܸ� |