��Ŀ����
�������Ϊ10 mL�����ʵ���Ũ����ͬ������NaOH��Һ�зֱ�ͨ��һ������CO2�õ���Һ
���ң���ס�������Һ�зֱ�μ�0.1mol��L-1���ᣬ��ʱ��Ӧ����CO2���(��״��) ���������������Ĺ�ϵ��ͼ��ʾ����������������ȷ���ǣ� ��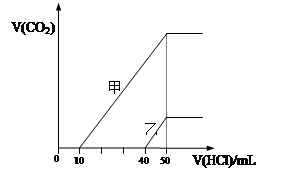
| A��ԭNaOH��Һ�����ʵ���Ũ��Ϊ0.5 mol��L-1 |
| B����0<V(HCl)<10mLʱ������Һ�з�����Ӧ�����ӷ���ʽΪ�� OH-+H��=H2O |
| C������Һ�к��е�������NaOH��NaHCO3 |
| D������Һ�еμ���������CO2��������ֵΪ112mL(��״��) |
A
�������������A��HCl����50mlʱ��ǡ�÷�Ӧ����NaCl������NaԪ�غ�ClԪ���غ㣬HCl�����ʵ�������NaOH�����ʵ���������c(NaOH)=0.05L��0.1mol/L��0.01L=0.5mol?L?1����ȷ��B�����ݼ�ͼ���֪������Һ����Na2CO3��NaHCO3������0<V(HCl)<10mLʱ������Һ�з�����Ӧ�����ӷ���ʽΪ��CO32?+H+��=HCO3?������C�������ҵ�ͼ���֪������Һ����NaOH��Na2CO3������D��������Һ�����HCl��� 40<V(HCl)<50mLʱ��������Ӧ��HCO3?+H+=CO2��+H2O��V(CO2)=0.01L��0.1mol/L��22.4L/mol=0.0224L=22.4mL������
���㣺���⿼�����ͼ��ͻ�ѧ����ʽ�ļ��㡣

����һ�����ʵ���Ũ�ȵ�NaOH��Һʱ�����������ҺŨ��ƫ�ߵ�ԭ����
| A������ʱ����Һ�� | B������NaOH�ѳ��� |
| C��δϴ���ܽ�NaOH������ձ� | D������ʱ����Һ�� |
��51.2gCu��ȫ��������Ũ�����У��ռ�������������� NO��N2O4��NO2���Ļ���ﹲ0.8mol����Щ����ǡ���ܱ�500mL2mol/LNaOH��Һ��ȫ���գ����ɵ�����Һ��NaNO3�����ʵ���Ϊ����֪��2NO2+2NaOH=NaNO3+NaNO2+H2O��NO+NO2+2NaOH=2NaNO2+H2O���� ��
| A��0.2mol | B��0.4mol | C��0.6mol | D��0.8mol |
��NAΪ�����ӵ�������ֵ������������ȷ����
| A��0.2mol����������ˮ������Ӧ���ɵ�H2������Ϊ0.3NA |
| B�����³�ѹ�£�0.1mol Na2O2��CO2��ȫ��Ӧת�Ƶ�����Ϊ0.1NA |
| C��50mL18.4mol��L��1Ũ����������ͭ�ȷ�Ӧ������SO2���ӵ���ĿΪ0.46NA |
| D��ij�ܱ�����ʢ��0.1molN2��0.3molH2,��һ�������³�ַ�Ӧ��ת�Ƶ��ӵ���ĿΪ0.6NA |
�����йذ���٤������˵����ȷ����
| A����0.2mol H2SO4��Ũ����������п��Ӧ����������ķ�����С��0.1NA |
| B������£�22.4L���Ȼ�̼��������������NA |
| C��0.1mol/L ��AgNO3��Һ�У������������������ĿΪ0.1NA |
| D�����267g����AlCl3��������3mol Cl2��54g������ |
����˵����ȷ����
| A��0.5mol SO2��11.2LCO2�����ķ�����Ŀһ����� |
| B��25���100��ʱ��ˮ��pH��� |
| C���к͵�����������ʵ���Ũ�ȵ�NaOH�Ͱ�ˮ�����ĵ�n(H2SO4)��� |
D��2SO2(g)��O2(g) 2SO3(g) ��4SO2(g)��2O2(g) 2SO3(g) ��4SO2(g)��2O2(g)  4SO3(g)�ġ�H��� 4SO3(g)�ġ�H��� |
NAΪ�����ӵ�����������������ȷ����
| A��10 g H218O���е�������Ϊ5NA |
| B��1 mol�ǻ�(-OH)���еĵ�����Ϊ10NA |
| C��1 mol �����μӷ�Ӧʱ������ת����Ŀһ��Ϊ2NA |
| D����״���£�11.2L���麬�еķ�����Ϊ0.5 NA |