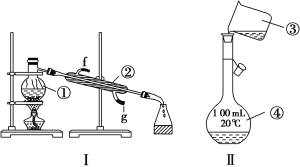
��Ŀ����
����Ŀ��ʵ������NaOH��������240mL 1.2mol/L��NaOH��Һ�������ش��������⣺
��1������240mL 1.2mol/L��NaOH��Һ
Ӧ��ȡNaOH������/g | ѡ������ƿ�Ĺ��/mL | ������ƿ���Ҫ�������������� |
_________ | ________ | _______ |
��2������ƿ����������������е�_____________________��
��Ũ�� �� �¶� ������ ��ѹǿ �ݿ̶���
��3������ʱ������ȷ�IJ���˳���ǣ���ĸ��ʾ��ÿ����ĸֻ����һ�Σ�___��
A��������ȴ��NaOH��Һ�ز�����ע��250mL������ƿ��
B������ƽȷ��ȡ�����NaOH����������������ˮ���ò���������������ʹ�����ܽ�
C����30mLˮϴ���ձ�2��3�Σ�ϴ��Һ��ע������ƿ����
D��������ƿ�ǽ����ߵ�ҡ��
E�����ý�ͷ�ιܼ�ˮ��ʹ��Һ����ǡ����̶�������
F������������ƿ��С�ļ�ˮ��ֱ��Һ��ӽ��̶�1��2cm��
G װ�����ñ�ǩ���Լ�ƿ����
��4���������Ƶ���ҺŨ��ƫ�͵���__��
A������NaOHʱ������ֽ�ϳ���
B������ǰ������ƿ������������ˮ
C��������ˮʱ���������˿̶���
D��������ƿ��ת����Һʱ������Һ����������ƿ����
E ����ƿ��ʹ��ǰ���
���𰸡�12 250ml�� �ձ�������������ͷ�ι� �ڢۢ� BACFEDG ACD
��������
��1������n=cv�����������Ƶ����ʵ������ٸ���m=nM���������������Ƶ��������������ƾ��и�ʴ�ԡ��׳��⣬Ӧ�����ձ���Ѹ�ٳ�����
��2������ƿ�ϱ����¶ȡ������Ϳ̶��ߣ�
��3��ʵ��������240mL 1.2mol/L NaOH��Һ�IJ���˳��Ϊ������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ��
��4������c=![]() ������Һ��������ʵ����ʵ����Ƿ����仯�������
������Һ��������ʵ����ʵ����Ƿ����仯�������
(1)ʵ����û��240mL��������ƿ������240mL 1.2mol/L��NaOH��Һ��Ҫѡ��250ml����ƿ����Ӧ��ȡNaOH������Ϊ��0.25L��1.2mol/L��40g/mol=12g����250ml����ƿ�⣬����Ҫ�IJ����������ձ�������������ͷ�ιܣ��ʴ�Ϊ��12��250ml���ձ�������������ͷ�ιܣ�
��2������ƿ�ϱ����¶ȡ������Ϳ̶��ߣ��ʴ�Ϊ���ڢۢ���
��3��ʵ��������240mL 1.2mol/L NaOH��Һ�IJ���˳��Ϊ������������ܽ⡢��ȴ����Һ��ϴ�ӡ����ݡ�ҡ�ȡ�װƿ��ǩ�����Խ�����ʵ�鲽��A��G��ʵ������Ⱥ��������ΪBACFEDG���ʴ�Ϊ��BACFEDG��
��4��A������NaOHʱ������ֽ�ϳ������������ƻ����տ����еĶ�����̼��ˮ���ᵼ��NaOH�����ʵ�����С��������ҺŨ�Ƚ�ƫ�ͣ�����ȷ��
B����ϡ�Ͷ��ɿ�֪��ϡ��ǰ��NaOH�����ʵ������䣬������ǰ������ƿ������������ˮ��������ҺŨ����Ӱ�죬�ʴ���
C��������ˮʱ���������˿̶��ߣ�������Һ�����ƫ��������ҺŨ��ƫ�ͣ�����ȷ��
D��������ƿ��ת����Һʱ������Һ����������ƿ���棬�ᵼ��NaOH�����ʵ�����С��������ҺŨ�Ƚ�ƫ�ͣ�����ȷ��
E������ƿ��ʹ��ǰ��ɶ�NaOH�����ʵ�������Һ�������Ӱ�죬��������ҺŨ����Ӱ�죬�ʴ���
ACD��ȷ���ʴ�Ϊ��ACD��

 ���ٴ�����ɽ����ϵ�д�
���ٴ�����ɽ����ϵ�д�����Ŀ�������ڼ��������¿ɻ�ԭ����ͭ����������ˮ�����⣬����̼�������ij��ѧС��������ͼװ��̽���䷴Ӧ���

[��������]��CO����������Һ��Ӧ��CO��2[Ag(NH3)2]����2OH��===2Ag����2NH4+��CO32����2NH3��
��Cu2OΪ��ɫ������Ag+��Ӧ���ܷ�����Ӧ��Cu2O��2H��===Cu2+��Cu��H2O��
��1��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
��2�������������װ�ô����ҵ�����˳��ΪA��__________________��(����ĸ���)
��3��ʵ���еμ�ϡ����IJ���Ϊ______________________________________________��
��4����֪��������к���CO����װ��C�пɹ۲쵽��������________________��װ��F������Ϊ_________________________________________��
��5������Ӧ������װ��D���Թ��й���ȫ����Ϊ��ɫ��
�����ʵ��֤����ɫ�����к���Cu2O��______________________________________________��
����֤����ɫ�������Ƿ���Cu����ͬѧ�������ʵ�飺��������ɫ�����м�������0.1mol��L1AgNO3��Һ��������Һ�������ݴ��жϺ�ɫ�����к���Cu����ͬѧ��Ϊ�÷�������������֤����ͬѧ�Ľ��ۣ������������¶Ա�ʵ�飬��ɱ������ݡ�
ʵ�鲽��(��Ҫ��д�������������) | Ԥ������ͽ��� |
__________________ | ���۲쵽��Һ����������֤����ɫ�����к���Cu�����۲쵽��Һ����������֤����ɫ�����к���Cu |