��Ŀ����
17��0.2molij��A����������ȫȼ�պ�����CO2��H2O��1.2mol���Իش���1����A�ķ���ʽΪC6H12
��2����ȡһ�����ĸ���A��ȫȼ�պ�����CO2��H2O��3mol������42_g��A�μ��˷�Ӧ��
��3������A����ʹ��ˮ��ɫ������һ��������������������ȡ����Ӧ����һ��ȡ����ֻ��һ�֣�����A�Ľṹ��ʽΪ

��4������A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4��������A�����еĽṹ��ʽΪ��CH3��3C-CH=CCH2����д1����
��5������A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ����5��ͬ���칹�壮
���� ��1����ȼ�����ɶ�����̼��ˮ����0.2mol��ȼ�����ɶ�����̼��ˮ��1.2mol��֪�л���ķ���ʽΪC6H12��
��2���ɷ�����ɿ�֪������CO2��H2O��3mol����Ϊ0.5mol����ȷ������������
��3������A����ʹ��ˮ��ɫ������һ�������£�����Cl2����ȡ����Ӧ����һ�ȴ���ֻ��һ�֣�˵�����в���̼̼˫����ӦΪ���������������飻
��4����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4������˵�����к���C=C�����У�����4��������3�֣���̼�ܽṹΪ���٢ڢ۴��ɷֱ�ͬʱ����˫������ ���Դ�ȷ��ϩ����
���Դ�ȷ��ϩ����
��5������A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ�����ʽΪC5H10������1��C=C˫�����ݴ���д����������ͬ���칹���жϣ�
��� �⣺��1��ij��A 0.2mol ����������ȫȼ�պ�����CO2��H2O��1.2mol��������к���N��C��=6��n��H��=12������ʽΪC6H12��
�ʴ�Ϊ��C6H12��
��2����ȡһ�����ĸ���A��ȫȼ�պ�����CO2��H2O��3mol���ɷ�����ɿ�֪��Ϊ0.5mol������Ϊ0.5mol����84g/mol��=42g��
�ʴ�Ϊ��42��
��3��C6H12ֻ��1�������Ͷȣ�����A����ʹ��ˮ��ɫ������Ϊ�����������У�������������ȡ����Ӧ����һ�ȴ���ֻ��һ�ֵ��ǻ����飬�� ��
��
�ʴ�Ϊ�� ��
��
��4����A��ʹ��ˮ��ɫ���ڴ��������£���H2�ӳɣ���ӳɲ��ᆳ�ⶨ�����к���4������˵�����к���C=C�����У�����4��������3�֣���̼�ܽṹΪ���٢ڢ۴��ɷֱ�ͬʱ����˫������ ����A�����еĽṹ��ʽΪ��CH3��3C-CH=CCH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2�ȣ�
����A�����еĽṹ��ʽΪ��CH3��3C-CH=CCH2��CH3-C��CH3��=C��CH3��-CH3��CH3CH��CH3��-C��CH3��=CH2�ȣ�
�ʴ�Ϊ����CH3��3C-CH=CCH2��
��5������A��һ��̼ԭ������ʹ��ˮ��ɫ��A��ͬϵ�����ʽΪC5H10������1��C=C˫��������������ͬ���칹��Ϊ��CH3CH2CH2CH=CH2��CH3CH2CH=CHCH3��CH2=C��CH3��CH2CH3����CH3��2C=CHCH3����CH3��2CHCH=CH2���ʴ�Ϊ��5��
���� ���⿼���л�����ƶϡ�ϩ�������ʡ�ͬ���칹�塢��ѧ����ȣ��Ѷ��еȣ�ע�⣨4���и��ݼӳɷ�Ӧԭ�������û�ԭC=C˫����������д��

| A�� | ��֪����ƽ�ⳣ����H2CO3��HClO��HCO3-����NaClO��Һ��ͨ������CO2��2ClO-+CO2+H2O=2HClO+CO32- | |
| B�� | ������������Һ�еμ�Ba��OH��2�����ԣ�H++SO42-+Ba2++OH-=BaSO4��+H2O | |
| C�� | Fe��NO3��3��Һ�м��������HI��Һ��2Fe3++2I-=2Fe2++I2 | |
| D�� | ��������ӵĵ��뷽��ʽ��HS-+H2O?H3O++S2- |
| A�� | ȫ�� | B�� | ֻ�Т� | C�� | �ں͢� | D�� | �ڢۢ� |
| A�� | 2�� | B�� | 4�� | C�� | 6�� | D�� | 8�� |
 ��1����ҵ�ϳɰ���һ�����淴Ӧ��N2��g��+H2��g��?2NH3��g����ͼ�����߲�����ͨ���ı仯ѧ��Ӧ�е������������ԭ���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1��573K�����������������=������
��1����ҵ�ϳɰ���һ�����淴Ӧ��N2��g��+H2��g��?2NH3��g����ͼ�����߲�����ͨ���ı仯ѧ��Ӧ�е������������ԭ���±�Ϊ��ͬ�¶��¸÷�Ӧ��ƽ�ⳣ�����ɴ˿���֪������T1��573K�����������������=������ | T/K | T1 | 573 | T3 |
| K | 1.00��107 | 2.54��105 | 1.88��103 |
��3���������ļ�̬��+2��+4�ۣ��ڻ��������ȶ��ļ�̬Ϊ+4�ۣ������Ƶ�SnCl2��ˮ��Һ���˹���SnCl2��ˮ�⣬����Ҫ�������������Sn��
��4������ȼ�Ͳ���ȫȼ��ʱ����CO���������밴���з�Ӧ��ȥCO��2CO��g��=2C��s��+O2��g������֪�÷�Ӧ�ġ�H��0�������������ܷ�ʵ�ֵ����ݣ�����ʵ�֣���Ϊ���H-T��S��0��
��5��Mg��OH��2����þ�Ρ��ͻ���Ϻ���ȼ������Ҫԭ�ϣ�
��֪��a��25��ʱ��KSP[Mg��OH��2]=4.0��10-38��b��Mg��OH��2��s��=MgO��s��+H2O��g����H=+81.5kJ•mol-1��
������������ȷ����BD��
A������±��ˮ�л��Mg��OH��2����ҵ��ѡ��NaOH��������
B��Mg��OH��2������ȼ�������ֽ�����������MgO���ǿ�ȼ��
C���ɼ���Mg��OH��2�õ�MgO���ٵ�����ڵ�MgO�ƽ���þ
D�������£�̼���Ժ�����þ������Ӧ
�����ָʾ�����������ɫ��pH��Χ���£�
| pH | ��8.0 | 8.0��9.6 | ��9.6 |
| ��ɫ | ��ɫ | ��ɫ | ��ɫ |
| A�� | CH3CH��CH3��2������״������ | B�� |  ���ڷ����廯���� ���ڷ����廯���� | ||
| C�� |  ����֬�������� ����֬�������� | D�� |  ���ڷ����� ���ڷ����� |
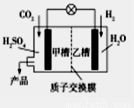
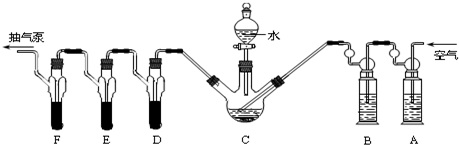
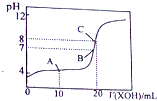 X��Y��Ԫ�����ڱ�ǰ20��Ԫ�أ���X��ԭ��������Y��4������գ�X��YҪ�þ����Ԫ�ط��ű�ʾ����
X��Y��Ԫ�����ڱ�ǰ20��Ԫ�أ���X��ԭ��������Y��4������գ�X��YҪ�þ����Ԫ�ط��ű�ʾ����