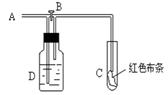
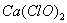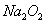��Ŀ����
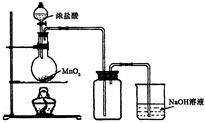
ijУ��ѧ�о���ѧϰС������������ʺ���;�����˵���������˽���������Ӧ�� ������Ư�ס�ˮ���ɱ����������ԭ����
(1) ��������ˮ������Ư�ס�������ԭ����_______����Ӧ�����ӷ���ʽΪ_______ ��
(2) ͨ��ʹ��Ư��Һ(NaClO��Һ)��Ư��������������������_____(д��һ���)��
(3) ��һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ (�����ķ�Ӧ��Ϊ���ȷ�Ӧ���������������к���Cl-�� ClO-��ClO3-���ֺ���Ԫ�ص����ӣ�����ClO-��ClO3-������ �ӵ����ʵ�����n)�뷴Ӧʱ�䣨t)�ı仯��������ͼ��ʾ��
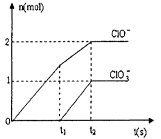
��O-t1ʱ���ڣ�Ca(OH)2��Cl2������Ӧ�Ļ�ѧ����ʽΪ_______��
��t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵĻ�ѧ����ʽΪ_______
�۸�ʯ�����к���Ca(OH)2��������_______g��
(1) ��������ˮ������Ư�ס�������ԭ����_______����Ӧ�����ӷ���ʽΪ_______ ��
(2) ͨ��ʹ��Ư��Һ(NaClO��Һ)��Ư��������������������_____(д��һ���)��
(3) ��һ������ʯ������ͨ��һ����������������ǡ����ȫ��Ӧ (�����ķ�Ӧ��Ϊ���ȷ�Ӧ���������������к���Cl-�� ClO-��ClO3-���ֺ���Ԫ�ص����ӣ�����ClO-��ClO3-������ �ӵ����ʵ�����n)�뷴Ӧʱ�䣨t)�ı仯��������ͼ��ʾ��

��O-t1ʱ���ڣ�Ca(OH)2��Cl2������Ӧ�Ļ�ѧ����ʽΪ_______��
��t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵĻ�ѧ����ʽΪ_______
�۸�ʯ�����к���Ca(OH)2��������_______g��
��9�֣�
��1��������ˮ��Ӧ����ǿ�����ԵĴ����ᣨ1�֣��� Cl2+H2O=HClO+H++ClO-��1�֣� ��
��2��NaClO���ȶ������ڴ�������䣨1�֣���
��3����2Ca(OH)2��2Cl2��Ca(C1O)2��CaCl2��2H2O��2�֣���
��10Ca(OH)2��10Cl2��2Ca(C1O)2��Ca(C1O3)2��7CaCl2��10H2O��2�֣���
��370 g ��2�֣�
��1��������ˮ��Ӧ����ǿ�����ԵĴ����ᣨ1�֣��� Cl2+H2O=HClO+H++ClO-��1�֣� ��
��2��NaClO���ȶ������ڴ�������䣨1�֣���
��3����2Ca(OH)2��2Cl2��Ca(C1O)2��CaCl2��2H2O��2�֣���
��10Ca(OH)2��10Cl2��2Ca(C1O)2��Ca(C1O3)2��7CaCl2��10H2O��2�֣���
��370 g ��2�֣�
���������(1)������ˮ��Ӧ���ɴ����ᣬ��������к�ǿ�������ԣ�����������ˮ������Ư�ס�������ԭ����������ˮ��Ӧ����ǿ�����ԵĴ����ᣬ��Ӧ�����ӷ���ʽΪCl2+H2O=HClO+H++ClO-��
(2)�����ж����Ҳ�����ʹ�ã���ͨ��ʹ��Ư��Һ(NaClO��Һ)��Ư��������������������NaClO���ȶ������ڴ�������䡣
(3) ����ͼ�������t1ʱ��û��ClO3-�������ɣ���O-t1ʱ���ڣ�Ca(OH)2��Cl2������Ӧ�Ļ�ѧ����ʽΪ2Ca(OH)2��2Cl2��Ca(C1O)2��CaCl2��2H2O��
����ͼ�������t2ʱ��ClO-��ClO3-�������ɣ���t2ʱ��Ca(OH)2��Cl2������Ӧ���ܵĻ�ѧ����ʽΪ10Ca(OH)2��10Cl2��2Ca(C1O)2��Ca(C1O3)2��7CaCl2��10H2O��
���ɻ�ѧ���̼���ø�ʯ�����к���Ca(OH)2��������370 g��
���������⿼��������������ʵ����֪ʶ����Ŀ�Ѷ��еȣ�����ѧ���Ի���֪ʶ�����ճ̶Ⱥͼ���������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ



 2KCl + 2MnCl2 + 5Cl2 �� + 8H2O
2KCl + 2MnCl2 + 5Cl2 �� + 8H2O