��Ŀ����
4������A-J�����ڱ��е�λ�ã���Ԫ�ط��Ż�ѧʽ�ش��������⣺| ������ | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 1 | A | |||||||
| 2 | D | E | G | I | ||||
| 3 | B | C | F | H | ||||
| 4 | J |
 ��
����2������Ԫ�أ���ѧ��������õ���Ne��ֻ�и��۶������۵���F����������ǿ�ĵ�����F2����ԭ����ǿ�ĵ�����Na��
��3������������ˮ���������ǿ����NaOH��������ǿ����HClO4�������Ե���Al��OH��3��
��4��A�ֱ���D��E��F�γɵĻ������У����ȶ�����NH3��
��5����C��D��G��H�У�ԭ�Ӱ뾶������Al��
���� ��Ԫ�������ڱ��е�λ�ÿ�֪��AΪH��BΪNa��CΪAl��DΪC��EΪN��FΪP��GΪF��HΪCl��JΪBr��IΪNe��Ȼ������Ԫ�ؼ��䵥�ʡ�����������������
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪��AΪH��BΪNa��CΪAl��DΪC��EΪN��FΪP��GΪF��HΪCl��JΪBr��IΪNe��
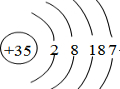
��1��JΪBr��ԭ������Ϊ35��ԭ�ӽṹʾ��ͼΪ ��
��
�ʴ�Ϊ�� ��
��
��2������Ԫ���У�ֻ��Ne����������Ϊ8��Ϊ�ȶ��ṹ����ѧ���ʲ����ã�ֻ�и��۶������۵���F��F�ķǽ�������ǿ����F2����������ǿ��Na�Ľ�������ǿ����ԭ����ǿ�ĵ�����Na���ʴ�Ϊ��Ne��F��F2��Na��
��3������Ԫ���У�Na�Ľ�������ǿ��������������ˮ����NaOH�ļ�����ǿ������Ԫ���е�����������ˮ������ֻ��Al��OH��3Ϊ���ԣ�Cl������������ˮ����HClO4������ǿ���ʴ�Ϊ��NaOH��HClO4��Al��OH��3��
��4��N��C��P��N�ķǽ�������ǿ����NH3���ȶ����ʴ�Ϊ��NH3��
��5�����Ӳ�Խ�࣬ԭ�Ӱ뾶Խ����ͬ���Ӳ�ʱ��ԭ���������ԭ�Ӱ뾶С����C��D��G��H�У�ԭ�Ӱ뾶������Al���ʴ�Ϊ��Al��
���� ���⿼��λ�á��ṹ�����ʣ��漰Ԫ�����ڱ���Ԫ�������ɣ�Ϊ��Ƶ���㣬��ȷԪ�������ڱ��е�λ���ǽ����Ĺؼ���Ȼ�����õ��ʡ����������������ɣ���Ŀ�ѶȲ���

| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
��SO2��CO2ת�������Ǿ����������츣�������Ч;����һ���õ绯ѧԭ������SO2��CO2ת������;�㷺�Ļ�����ƷH2SO4��CH3OH����װ����ͼ��ʾ��

��1����װ�õĹ���ԭ����ԭ��أ����ԭ��ء����ء���
��2����AΪCO2��BΪH2��CΪCH3OH����ͨ��H2-��Ϊ����
��3����AΪSO2��BΪO2��CΪH2SO4����A���ĵ缫��ӦʽΪSO2+2H2O-2e-=SO42-+4H+��
�����ǵؿ���ϡ��Ԫ�أ���ҵ�����ķ��ϣ���Ҫ�ɷ֣������ڡ�̼��ͭ�����Ͻ𣩻��վ�������һ�ֹ����������£�
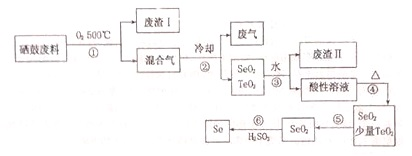
��֪��
| �������� | �۵� | �е� | ���� | �ܽ��� |
| SeO2 | 340�� | 684�� | 315�� | ������ˮ |
| TeO2 | 733�� | 1260�� | 450�� | ������ˮ |
��2��������Ҫ�ɷ���CO2��
��3������ܵķ�Ӧ����ʽ��H2SeO3$\frac{\underline{\;\;��\;\;}}{\;}$SeO2+H2O������ķ�Ӧ����ʽ��2H2SO3+SeO2=Se+2H2SO4��
��4�����ݱ������ݣ�������з���SeO2��TeO2����ѷ��������������¶ȿ�����315�浽450��֮�䣮
| A�� | V��NaOH��=0ʱ��c��H+��=1��10-2 mol/L | |
| B�� | V��NaOH����10 mLʱ�����ܴ���c��Na+��=2c��C2O42-��+c��HC2O4-�� | |
| C�� | V��NaOH��=10mLʱ��c��H+��=1��10-7 mol/L | |
| D�� | V��NaOH����10 mLʱ��c��Na+����c��HC2O4-����c��C2O42-�� |
| A�� | 5.04L | B�� | 6.72L | C�� | 20.16L | D�� | 40.32L |
| A�� | R�������ﶼ������ˮ | |
| B�� | R������������Ӧ��ˮ���ﶼ��H2RO3 | |
| C�� | R�Ƿǽ���Ԫ�� | |
| D�� | R�������ﶼ����NaOH��Һ��Ӧ |
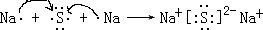 ��
�� һ��������������ԭ��Ӧ�ķ���ʽ���Բ�д���������뷴Ӧʽ����һ����������Ӧʽ����һ���ǻ�ԭ��Ӧʽ����2Fe3++Cu�T2Fe2++Cu2+�IJ�д����ǣ�
һ��������������ԭ��Ӧ�ķ���ʽ���Բ�д���������뷴Ӧʽ����һ����������Ӧʽ����һ���ǻ�ԭ��Ӧʽ����2Fe3++Cu�T2Fe2++Cu2+�IJ�д����ǣ�