��Ŀ����
����Ŀ��ij��ȤС����ʵ�������Ҵ���Ũ������廯�ƺ�ˮ��Ϸ�Ӧ���Ʊ������飬��̽������������ʡ��й����ݼ��±���
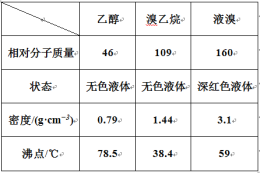
I. ��������Ʊ�
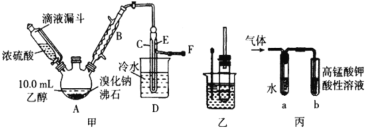
��Ӧԭ�����£�ʵ��װ������ͼ������װ�á��г�װ�þ�ʡ�ԣ���
H2SO4+NaBr ![]() NaHSO4+HBr�� CH3CH2OH+HBr
NaHSO4+HBr�� CH3CH2OH+HBr ![]() CH3CH2Br+H2O
CH3CH2Br+H2O
��1�� ͼ����A ����������_____��ͼ��B �����ܵ�����Ϊ_____��
��2�� ��ͼ����A �����¶ȹ���Ũ�����Ũ�ȹ�����ʹ C ���ռ����Ĵֲ�Ʒ�ʳ�ɫ��ԭ����A �з����˸���Ӧ������_____��F ���ӵ���ͨ��ϡNaOH ��Һ�У���Ŀ����Ҫ������_____��β����ֹ��Ⱦ����
II. ���������ʵ�̽��
����ͼʵ��װ����֤����������ʣ�
��3�� �������Թ��ڼ��� 10mL6mol��L ��1NaOH ��Һ�� 2mL �����飬�����ã�Һ��ֲ㣬ˮԡ���ȡ��ù����еĻ�ѧ����ʽΪ_______��
��4�� ���������Թ���� NaOH ��Һ����NaOH �Ҵ���Һ��Ϊ֤������Ϊ��ϩ�������ɵ�����ͨ����ͼ��װ�á�a �Թ��е�ˮ��������_______������ a �Թܣ������ɵ�����ֱ��ͨ�� b �Թ��У��� b�е��Լ�����Ϊ _____��
���𰸡�������ƿ �������������ӷ�Ӧ�������� Br2 SO2��Br2��HBr CH3CH2Br+NaOH![]() CH3CH2OH+NaBr �����Ҵ� ��ˮ(�����CCl4��Һ)
CH3CH2OH+NaBr �����Ҵ� ��ˮ(�����CCl4��Һ)
��������
װ�ü���ͨ��Ũ������廯�Ʒ�Ӧ�����廯�⣬Ȼ�������廯����Ҵ���Ӧ�Ʊ������飬��Ũ�������ǿ�����ԣ����ܽ��廯�����������嵥�ʣ�ʹ��Һ�ʳ�ɫ�������ܽ�������������Ȼ��������ˮ��ȴ�����ռ������飻�������ܹ�������������Һ����ȡ����Ӧ�����Ҵ����������ܹ���NaOH�Ҵ���Һ�����������·�����ȥ��Ӧ������ϩ����ϩ�ܹ�ʹ���Ը��������Һ��ɫ������Ҫע���ȥ��ϩ�����л��е��Ҵ�����Ҫ��ȥ�Ҵ����Դ˽���⡣
I����1������AΪ������ƿ���Ҵ��ӷ��������ܵ�����Ϊ�������������ӷ�Ӧ�������ʣ�
��2����ͼ����A�����¶ȹ���Ũ�����Ũ�ȹ�����ʹC���ռ����Ĵֲ�Ʒ�ʳ�ɫ�����ǵ�Ũ�������ǿ�����ԣ���Ӧ�¶ȹ���ʹ��Ӧ���ң�������ɫ��Br2��������Ӧ�Ļ�ѧ����ʽΪ��2HBr+H2SO4(Ũ)=Br2+SO2+2H2O��F���ӵ���ͨ��ϡNaOH��Һ�У����ǵ���Ӧ����SO2��Br2��HBr���壬����Ⱦ������Ӧ��NaOH��Һ���գ�����Ŀ����Ҫ�ǣ�����SO2��Br2��HBr����ֹ��Ⱦ������
II����3�������鲻����ˮ��һ��ʼ���ֲַ㣬������NaOH��Һˮԡ���ȷ�����Ӧ������NaBr���Ҵ�����ӦΪ��CH3CH2Br+NaOH![]() CH3CH2OH+NaBr��
CH3CH2OH+NaBr��
��4��Ϊ֤����������NaOH�Ҵ���Һ�з�Ӧ���������Ϊ��ϩ�������ɵ�����ͨ����ͼ��װ�ã����ŷ�Ӧ�ķ�������������ϩ�п��ܻ�����Ҵ����Ҵ�Ҳ��ʹ���Ը��������Һ��ɫ����Ӧ�ȳ�ȥ���е��Ҵ�������֤��ϩ������a�Թ��е�ˮ�������ǣ������Ҵ�������a�Թܣ�b�Թ��е��Լ�ӦΪ������ϩ��Ӧ�������Ҵ���Ӧ���ɿ�����ˮ(�����CCl4��Һ)���ʴ�Ϊ�������Ҵ�����ˮ(�����CCl4��Һ)��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ijͬѧ����ͼ��ʾʵ��װ��̽��ͭ��Ũ����ķ�Ӧ����¼ʵ���������������˵����ȷ����
�Թ� | �� | �� | �� | �� |
ʵ������ | ��Һ��Ϊ��ɫ���а�������ɫ������� | �д�����ɫ�������� | ��������ɫ�������� | Ʒ����Һ��ɫ |

A.���а�ɫ������BaSO3
B.���п����в���Ũ����ӷ���
C.Ϊ��ȷ�����а�ɫ�����Ƿ�Ϊ����ͭ��������ȴ����Թ���ע��ˮ����
D.ʵ��ʱ������װ����ͨ������N2���ټ����Թܢ٣�ʵ������