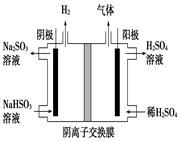
��Ŀ����
������һ�����͵���ɫ��Դ������һ����Ҫ�Ļ���ԭ�ϡ�
��1������ȼ����ֵ�ߡ�ʵ���ã��ڳ��³�ѹ�£�1 g H2��ȫȼ������Һ̬ˮ���ų�142.9 kJ��������H2ȼ���ȵĻ�ѧ����ʽΪ ��
��2�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�����ʽ���£�N2(g)��3H2(g) 2NH3(g)����H����92.4 kJ/mol
2NH3(g)����H����92.4 kJ/mol
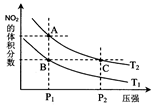
�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ�������(���ı�N2��H2��NH3����)����Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��ͼ��t1ʱ����ƽ���ƶ������������� ���б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����

���¶�ΪT ��ʱ����2 a mol H2��a mol N2����0.5 L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50%����Ӧ��ƽ�ⳣ��Ϊ
��1������ȼ����ֵ�ߡ�ʵ���ã��ڳ��³�ѹ�£�1 g H2��ȫȼ������Һ̬ˮ���ų�142.9 kJ��������H2ȼ���ȵĻ�ѧ����ʽΪ ��
��2�������Ǻϳɰ�����Ҫԭ�ϣ��ϳɰ���Ӧ���Ȼ�����ʽ���£�N2(g)��3H2(g)
 2NH3(g)����H����92.4 kJ/mol
2NH3(g)����H����92.4 kJ/mol�ٵ��ϳɰ���Ӧ�ﵽƽ��ı�ijһ�������(���ı�N2��H2��NH3����)����Ӧ������ʱ��Ĺ�ϵ����ͼ��ʾ��ͼ��t1ʱ����ƽ���ƶ������������� ���б�ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����

���¶�ΪT ��ʱ����2 a mol H2��a mol N2����0.5 L�ܱ������У���ַ�Ӧ����N2��ת����Ϊ50%����Ӧ��ƽ�ⳣ��Ϊ
��1�� H2(g)�� 1/2O2(g)��H2O(l)����H����285.8 kJ/mol
��2��������ѹǿ��t2��t3����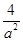
��2��������ѹǿ��t2��t3����
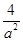
�����������1��1g���������ʵ�����0.5mol��ȼ��0.5mol�����ų�142.9kJ����������ȼ��1mol�����ų�285.8kJ����������������ȼ���Ȼ�ѧ��Ӧ����ʽΪ��H2��g��+1/2O2��g��=H2O��1����H=-285.8kJ/mol��
��2��N2��g��+3H2��g��?2NH3��g������H=-92.4kJ/mol����Ӧ�Ƿ��ȷ�Ӧ����Ӧǰ�����������С�������ݷ�Ӧ������ʱ��Ĺ�ϵͼ�������t1ʱ���淴Ӧ���ʶ�����ƽ�������ƶ�������ƽ���ƶ�����������������ѹǿ����ʾƽ��������NH3�ĺ�����ߵ�һ��ʱ����t2-t3��
�� N2��g��+3H2��g��?2NH2��g��
��ʼ 2amol/L 4amol/L 0
��Ӧ amol/L 3amol/L 2amol/L
ƽ�� amol/L amol/L 2amol/L
���Ի�ѧƽ�ⳣ��K=(2amol/L)2/(amol/L)?(amol/L)3=4/a2��L/mol��2��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 N2��g��+ CO2��g������H= Q kJ?mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£�
N2��g��+ CO2��g������H= Q kJ?mol-1����T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ�Ũ�����£� SO3��g����NO��g��
SO3��g����NO��g��
 CO(g)+H2(g)����÷�Ӧ��ƽ�ⳣ������ʽΪ ��
CO(g)+H2(g)����÷�Ӧ��ƽ�ⳣ������ʽΪ ��