��Ŀ����
����Ŀ��ij��ѧ��ȤС��ⶨijFeCl3��Ʒ��������FeCl2���ʣ�����Ԫ�ص�����������ʵ���Ұ����²�����У�
������a g��Ʒ�������ձ��У�
�����������������������ˮ��ʹ��Ʒ�ܽ⣬Ȼ��ȷ���Ƴ�250mL��Һ��
��ȷ��ȡ25.00mL����������õ���Һ�������ձ��У�������������ˮʹ��Ӧ��ȫ��
������Ѹ�ټ��������ˮ����ֽ��裬ʹ������ȫ��
�����ˣ�ϴ�ӳ�����
��������ת�Ƶ������ڣ����ȡ����裬ֱ������ȫ���ɺ��ɫ��Ϊ����ɫ���ڸ���������ȴ�����º�����
���ظ����ϲ�������������γƵõ�����������0.1gΪֹ��
����������������ش�
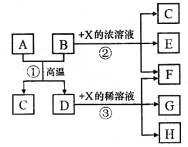
(1)��ͼ��ʾ�����У���ʵ�鲽���٢ڢ��б����õ���������E��F��__________������ĸ����
(2)���������������������________________________________________��
(3)д���������з�����Ӧ�Ļ�ѧ����ʽΪ______________________________��
(4)��������ϴ����ϴȥ�����ڳ����ϵ�________��д���ӷ��ţ���ϴ�ӳ����IJ���_________��
(5)������������W1����������Ⱥ������������W2������Ʒ����Ԫ�ص���������Ϊ__________��
���𰸡�C G ��ֹFeCl3��FeCl2ˮ�� FeCl3+3NH3��H2O��Fe(OH)3��+3NH4Cl NH4+��Cl����OH�� ��©����ע������ˮ��ʹˮ��û����������ˮ��Ȼ���º��ظ�����2��3�� ![]()
��������
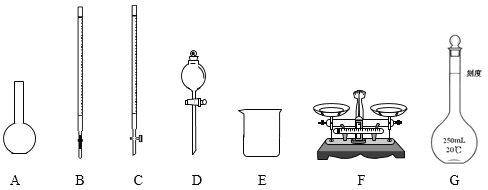
(1)�����ʱ��Ҫ��ƽ��ҩ�ף��ܽ�ʱ��Ҫ�ձ���������������һ�����ʵ���Ũ�ȵ���Һʱ��Ҫ��Ͳ����ͷ�ιܡ��ձ�����������һ����������ƿ��ȷ��ȡ��Һȷ��������ʽ�ζ��ܡ�ϴ���ʴ�Ϊ��CG��
(2)��FeCl3��FeCl2�ܷ���ˮ�⣬���������ܹ�������ˮ��̶ȣ��ʴ�Ϊ����ֹFeCl3��FeCl2ˮ�⣻
(3)������м��백ˮ��������ӦFeCl3+3NH3��H2O��Fe(OH)3��+3NH4Cl���ʴ�Ϊ��FeCl3+3NH3��H2O��Fe(OH)3��+3NH4Cl��
(4)����Һ�к��а�ˮ���Ȼ�泥�����������NH4+��Cl��OH��ϴ�ӳ����ķ���Ϊ��©����ע������ˮ��ʹˮ��û����������ˮ��Ȼ������ظ�����23�Σ��ʴ�Ϊ��NH4+��Cl��OH����©����ע������ˮ��ʹˮ��û����������ˮ��Ȼ������ظ�����23�Σ�
(5)����Ԫ�������غ㣬������ɫ�����е���������Ʒ������Fe2O3����Ԫ�ص�����Ϊ��W2-W1��g��![]() ��
��![]() =7��W2-W1��g����Ʒ����Ԫ�ص���������Ϊ��
=7��W2-W1��g����Ʒ����Ԫ�ص���������Ϊ��![]() ���ʴ�Ϊ��
���ʴ�Ϊ��![]() ��
��