��Ŀ����
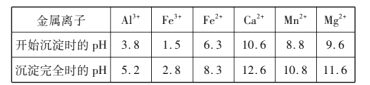
����Ŀ���������̿���Ҫ�ɷ���MnCO3��������A12O3��Fe2O3��FeO��CaO��MgO�ȣ�Ϊԭ���Ʊ�MnO2�Ĺ����������£�
��֪����25��ʱ��![]()
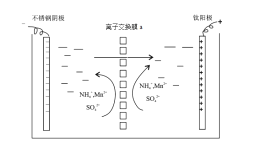
����ؽ�������![]() �γ������������pH��Χ���£�
�γ������������pH��Χ���£�
��1��������1���м�������MnO2��������____��Ӧ������ҺpH��С��____��
��2��������2������ҪĿ�Ľ�Ca2+��Mg2+ת��Ϊ��Ӧ�ķ������������ȥ����ȥCa2+�����ӷ���ʽΪ____���÷�Ӧ��ƽ�ⳣ��Ϊ____��
��3����������������Mn��OH��2'MnCO3���������ӷ���ʽΪ____����ĸҺ�������ȵ�ϵ�в�����ɷ�����____������ѭ��ʹ�á�
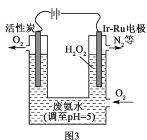
��4����MnSO4-��NH4��2SO4Ϊ�������Һ��������ͼװ�ÿ�ͬʱ�Ʊ������̺�MnO2�����ӽ���ĤaΪ____�������缫��ӦʽΪ____��
���𰸡���Fe2+����ΪFe3+ 5.2 MnF2(s)+ Ca2+(aq)![]() Mn2+(aq)+ CaF2(s) 3.53��107 2Mn2++3CO32-+2H2O= Mn(OH)2��MnCO3+2HCO3- ���� �����ӽ���Ĥ Mn2+-2e-+2H2O=MnO2+4H+
Mn2+(aq)+ CaF2(s) 3.53��107 2Mn2++3CO32-+2H2O= Mn(OH)2��MnCO3+2HCO3- ���� �����ӽ���Ĥ Mn2+-2e-+2H2O=MnO2+4H+
��������
��������ͼ��������������Һ�к���Mn2+��Al3+��Fe2+��Fe3+��Mg2+��Ca2+����������MnO2��Fe2+����ΪFe3+������pH����ȥAl3+��Fe3+������MnF2��ȥMg2+��Ca2+����Һ��ͨ��̼�����Һ���̡�
��1���������̾��������ԣ�������1���м�������MnO2�������ǰ�Fe2+����ΪFe3+��ΪʹAl3+��Fe3+������ȫ��Ӧ������ҺpH��С��5.2��
��2��������2������ҪĿ�Ľ�Ca2+��Mg2+ת��Ϊ��Ӧ�ķ������������ȥ����ȥCa2+�����ӷ���ʽΪMnF2(s)+ Ca2+(aq)![]() Mn2+(aq)+ CaF2(s)���÷�Ӧ��ƽ�ⳣ��
Mn2+(aq)+ CaF2(s)���÷�Ӧ��ƽ�ⳣ��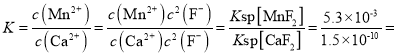 3.53��107��
3.53��107��
��3������������MnCl2��̼��立�Ӧ����Mn(OH)2��MnCO3�����ݵ���غ㡢�����غ㣬��Ӧ�����ӷ���ʽΪ2Mn2++3CO32-+2H2O= Mn(OH)2��MnCO3+2HCO3-����ĸҺ�������Ȼ�泥������ȵ�ϵ�в�����ɷ���������������ѭ��ʹ�á�
��4����MnSO4-(NH4)2SO4Ϊ�������Һ�Ʊ������̺�MnO2������ͼʾ������������Ĥ�������ƶ������ӽ���Ĥa�����ӽ���Ĥ������������ʧ���ӷ���������Ӧ���ɶ������̣��缫��ӦʽΪMn2+-2e-+2H2O=MnO2+4H+��