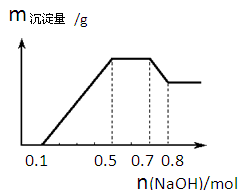
��Ŀ����
ά����C������ʽΪC6H8O6�����н�ǿ�Ļ�ԭ�ԣ������ڿ������ױ��������京����ͨ������������Һ������֪��Һ��I2��Һ���еζ����÷�Ӧ�Ļ�ѧ����ʽ���£�
C6H8O6+I2 C6H6O6+2HI
C6H6O6+2HI
�����ⶨij��Ʒ��ά����C�ĺ���������IJ��輰��õ��������¡�ȡ10mL 6mol��L��1CH3COOH������100mL����ˮ������Һ������к������ȴ����ȷ��ȡ0��2000g��Ʒ���ܽ���������ȴ����Һ�У�����1mL����ָʾ����������Ũ��Ϊ0��05000mol��L��1��I2��Һ���еζ���ֱ����Һ�е���ɫ��������Ϊֹ��������21��00mLI2��Һ��
��1��Ϊ�μ����CH3COOHϡ��ҺҪ�Ⱦ���С���ȴ�����ʹ�ã�
��2��������Ʒ��ά����C������������
C6H8O6+I2
 C6H6O6+2HI
C6H6O6+2HI �����ⶨij��Ʒ��ά����C�ĺ���������IJ��輰��õ��������¡�ȡ10mL 6mol��L��1CH3COOH������100mL����ˮ������Һ������к������ȴ����ȷ��ȡ0��2000g��Ʒ���ܽ���������ȴ����Һ�У�����1mL����ָʾ����������Ũ��Ϊ0��05000mol��L��1��I2��Һ���еζ���ֱ����Һ�е���ɫ��������Ϊֹ��������21��00mLI2��Һ��
��1��Ϊ�μ����CH3COOHϡ��ҺҪ�Ⱦ���С���ȴ�����ʹ�ã�
��2��������Ʒ��ά����C������������
��1�������Ϊ�˳�ȥ��Һ����Һ��O2������ά����C��O2��������ȴ��Ϊ�˼����ζ�������ά����C��Һ���Ͽ����Ӵ�ʱ���������ٶȡ�
��2��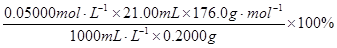 =92��40%
=92��40%
��2��
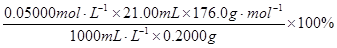 =92��40%
=92��40%���������ά����C������ʽΪC6H8O6�����н�ǿ�Ļ�ԭ�ԣ������ڿ������ױ������������Ϊ�˳�ȥ��Һ����Һ��O2������ά����C��O2��������ȴ��Ϊ�˼����ζ�������ά����C��Һ���Ͽ����Ӵ�ʱ���������ٶȣ�
�⣺����Ʒ��ά����C����������Ϊx������
C6H8O6 + I2
 C6H6O6 + 2HI
C6H6O6 + 2HI 176 1
0��2000x 0��05000mol��L��1��0.021L
X=
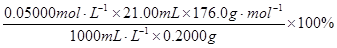 =92��40%
=92��40%�����������˻�ѧ�����е�Ũ�ȵļ��㣬���Ǻ��ѣ�Ҫ��ѧ�����������ö�����ϵ���м��㣬����ѧ���ļ���������

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ