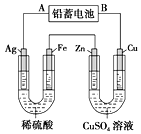
��Ŀ����
����Ŀ�����и���ʵ������Һ���ȱ���ǵ��ǣ�������
A. 0.1mol��LNa2S2O3��H2SO4��5mL����ˮ5mL����Ӧ�¶�10��
B. 0.1mol��LNa2S2O3��H2SO4��5mL����ˮ10mL����Ӧ�¶�10��
C. 0.1mol��LNa2S2O3��H2SO4��5mL����ˮ5mL����Ӧ�¶�30��
D. 0.2mol��LNa2S2O3��H2SO4��5mL����ˮ10mL����Ӧ�¶�30��
���𰸡�D
��������
����Na2S2O3+H2SO4=Na2SO4+SO2��+H2O����Ӧ��Ũ��Խ��Ӧ�¶�Խ�ߣ���Ӧ���ʾͻ�Խ�죬��Ӧ��ѡ��D

 ��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�
��Ǭ����������ҵ���ּ����ӱ����������ϵ�д�����Ŀ���۹�������Ŀǰ���߷����������о����ȵ㣬һ���øֹܳ��ķ���������Ҫ�ɷ�Fe3O4������C��SiO2��Ϊԭ���Ʊ����������£�

��֪����һ���¶������ʱFe3+��pH=2��ʼ������pH=3.7������ȫ
��1�����������С����顱��Ŀ����___________________________________________________��
��2��������������˵���Ũ�ȡ�Һ�̱ȡ�����¶ȡ��������ȣ���������¶ȶ�����ȡ�ʵ�Ӱ�����±���ʾ��
�¶ȡ� | 40 | 60 | 80 | 100 | 120 |
����ȡ�� | 50 | 62 | 80 | 95 | 85 |
����д�����������Fe3O4���������ӷ�Ӧ����ʽ__________________________________��
���������ʱӦ������Һ��pH____________����ԭ����_________________________________��
�۵�����¶ȳ���100��ʱ������ȡ�ʷ�����С����ԭ����___________________��
��3���������˲������Һ����Ҫ�ɷ�Ϊ____________���ѧʽ����
��4��Fe3+Ũ�ȶ�������������SnCl2��Fe3+��ԭΪFe2+�������������£�����K2Cr2O7����Һ�ζ�Fe2+��Cr2O72-����ԭΪCr3+�����õζ���Ӧ�����ӷ���ʽΪ____________��