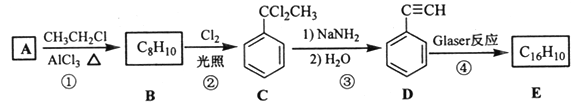
��Ŀ����
����Ŀ���������ֽ�ˮ�����������һ����Ҫ���л��ϳ�ԭ�ϡ�ij��ѧС����ˮ���ᣨ![]() ���ͼ״������Դ������ºϳ�ˮ�����������������ʡ�
���ͼ״������Դ������ºϳ�ˮ�����������������ʡ�
ʵ�鲽�裺
������ͼ����������ƿ�м���13.8g (0.1mol��ˮ�����24 g(30 mL��0.75 mol���״����������м���Լ10 mL�ױ�(�ױ���ˮ�γɵĹ�����е�Ϊ85������ʵ���м���ױ�����ˮ����������С�ĵؼ���5 mLŨ���ᣬҡ�����ȣ�����1��2����ʯ����װ��ʵ��װ�ã���85~95���º��¼��ȷ�Ӧ1.5Сʱ��
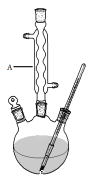
������װ����ȴ������״���Ȼ��ת������Һ©��������������ˮ��5%NaHCO3��Һ��ˮϴ�ӣ��ֳ��IJ������������ˮMgSO4���壬���˵õ�������
�������������������ռ�221����224������֣���ˮ�������9.12g��
��������������
���� | ������ | ��ɫ״̬ | ����ܶ� | �۵�(���� | �е�(���� |
ˮ������� | 152 | ��ɫҺ�� | 1.18 | -8.6 | 224 |
ˮ���� | 138 | ��ɫ���� | 1.44 | 158 | 210 |
�״� | 32 | ��ɫҺ�� | 0.792 | -97 | 64.7 |
�����������Ϣ�ش��������⣺
��1������A�������� �������ʯ�������� �������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������ ��
��2���Ʊ�ˮ�������ʱ������ʵļ��ȷ����� ��
��3��ʵ���м���ױ��Ժϳ�ˮ��������������� ��
��4����Ӧ����������״����õķ����� ��
��5��ʵ���м�����ˮ����þ�������� ��
��ʵ��IJ���Ϊ (������λ��Ч��������
���𰸡���1�������� ��ֹ���� ֹͣ���ȣ�����ȴ�����
��2��ˮԡ����
��3������ױ���ˮ������ʹƽ�������ƶ����Ӷ���߷�Ӧ�IJ���
��4�������ˮϴ��Һ
��5����ˮ����� 60%
��������
�����������1������A�������������ܣ�����ʱΪ�˷�ֹ����Ӧ�����ʯ�������Ⱥ���δ�ӷ�ʯ��Ӧ��ȡ����ȷ������ֹͣ���ȣ�����ȴ����룻
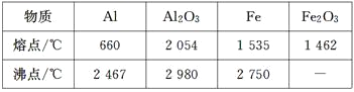
��2���Ʊ�ˮ�������ʱ�����ڼ״��ķе���64.7�棬Ϊ�˱�֤��Ӧ�ﲻ�ݳ�����������ʵļ��ȷ�����ˮԡ���ȣ�
��3����Ӧ����ˮ���������ˮ��ˮ������������ڼױ�������ʵ���м���ױ���ˮ������ʹƽ�������ƶ����Ӷ���߷�Ӧ�IJ��ʣ�
��4���״��ķе���64.7�棬�����������룬Ҳ�������ü״�������ˮ���л��ﲻ����ˮ�����ʣ���ˮϴ��Һ�����룻
��5��ʵ���м�����ˮ����þ����������ˮ����������ã�����0.1molˮ�����0.75 mol�״����״���������ˮ����Ϊ�����㣬�����ϲ���0.1mol��15.2gˮ���������ʵ�ʵ�ˮ�������9.12g�����Ա�ʵ��IJ���Ϊ9.12/15.2��100%=60%��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ��ij��ѧ��ȤС�飬ͨ����ͼװ�ý���������ͭ��Ӧ��ʵ��̽����
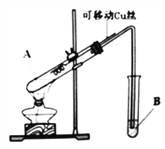
��������裺
����һ������ʱ��ϡH2SO4��Cu�ܷ�Ӧ��
�����������ʱ��ŨH2SO4��Cu�ܷ�Ӧ��
�����ʵ�飺
ʵ���� | ʵ����� | ʵ������ |
1 | ��Cu˿��ϡH2SO4�Ӵ������� | �Թ�B����Һ�����Ա仯 |
2 | ��Cu˿��ŨH2SO4�Ӵ������� | �Թ�B����Һ��ɫ |
ʵ��ǰ���Թ�Bʢ�ŵ���_______________��Һ���Ʒ�족��̪������
��ʵ����ۣ�ͨ������ʵ�飬˵��Ũ�������____________________����д��ĸ���ţ���
A.ǿ������ B.��ˮ�� C.��ˮ��
����������ۣ�
����ͬѧ��Ϊ��װ�ò����ƣ�Cu��ŨH2SO4��Ӧ������һ�ִ�����Ⱦ���������________�����������Թ�B����һ������������ý�������������Һ__________������ĸ��ţ���
A��ϡ���� B.NaOH��Һ C��NaCl��Һ
�ڸ���ȤС����̽��Cu��ŨH2SO4��Ӧʱ��������5.0g���ƶ�Cu˿��������ŨH2SO4�У���ַ�Ӧ��Cu˿ʣ��3.4g�����Թ�A��Һ��ϡ�ͳ�100ml��Һ������Һ��Cu2�����ʵ���Ũ��Ϊ_____mol��L��1 ��