��Ŀ����
18��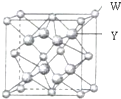 X��Y��Z��W����Ԫ�طֱ�λ�����ڱ����������ڵ����ڣ���ԭ������������X��Y���⻯�ﶼ��ͬ����һ����Ԫ���⻯��ķе�ߣ�����ͬ������ȴ������ߵģ�Z��ͬ����Ԫ�������Ӱ뾶��С��Ԫ�أ�Wԭ�ӵļ۵��Ӳ����������˶�״̬��ͬ�ĵ��ӣ���ش��������⣺
X��Y��Z��W����Ԫ�طֱ�λ�����ڱ����������ڵ����ڣ���ԭ������������X��Y���⻯�ﶼ��ͬ����һ����Ԫ���⻯��ķе�ߣ�����ͬ������ȴ������ߵģ�Z��ͬ����Ԫ�������Ӱ뾶��С��Ԫ�أ�Wԭ�ӵļ۵��Ӳ����������˶�״̬��ͬ�ĵ��ӣ���ش��������⣺��1��X��Y����Ԫ�ص�Ԫ�ط����ǣ�N��F��X��Y�����γ�һ�ֹ��ۻ������������Ԫ���������������ﵽ8��������ӵĿռ乹���ǣ������Σ�����ԭ�ӵ��ӻ���ʽ�ǣ�sp3�����ֻ����ﲻ���������γ������ӵ�ԭ����F�ĵ縺�Ա�N��N-F�ɼ����Ӷ���Fƫ�ƣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ������NF3�����������γ������ӣ�
��2��X���⻯��������ˮ��ԭ���ǣ�������ˮ��Ӧ������������ˮ�γ������������ˮ���Ǽ��Է��ӣ��������ܣ��ʰ���������ˮ��
��3��Z�ĵ����Ų�ʽ�ǣ�1s22s22p63s23p1������Ԫ��ԭ�ӵ���Χ�����Ų��������ɽ����ڱ��ֳ������������Z����p����
��4��W��Y�γɵĻ�����ľ����߳�Ϊa pm�������к���4��W���ӡ�W���ӵ���λ����8��������ܶ���$\frac{4��78}{��a��1{0}^{-10}��^{3}��{N}_{A}}$g•cm-3����ֻҪ������ʽ�����ؼ������ֵ�������ӵ�������ֵΪNA����
���� X��Y��Z��W����Ԫ�طֱ�λ�����ڱ����������ڵ����ڣ���ԭ������������X��Y���⻯�ﶼ��ͬ����һ����Ԫ���⻯��ķе�ߣ�����֮����������X��Y���ڵڶ����ڣ�����ͬ������ȴ������ߵģ�����֪XΪN��YΪF����Z���ڵ������ڡ�W���ڵ������ڣ�Z��ͬ����Ԫ�������Ӱ뾶��С��Ԫ�أ���ZΪAl��Wԭ�ӵļ۵��Ӳ����������˶�״̬��ͬ�ĵ��ӣ����ڢ�A��WΪCa���ݴ˽��
��� �⣺X��Y��Z��W����Ԫ�طֱ�λ�����ڱ����������ڵ����ڣ���ԭ������������X��Y���⻯�ﶼ��ͬ����һ����Ԫ���⻯��ķе�ߣ�����֮����������X��Y���ڵڶ����ڣ�����ͬ������ȴ������ߵģ�����֪XΪN��YΪF����Z���ڵ������ڡ�W���ڵ������ڣ�Z��ͬ����Ԫ�������Ӱ뾶��С��Ԫ�أ���ZΪAl��Wԭ�ӵļ۵��Ӳ����������˶�״̬��ͬ�ĵ��ӣ����ڢ�A��WΪCa��
��1��������������֪��XΪNԪ�ء�YΪFԪ�أ�X��Y�����γ�һ�ֹ��ۻ������������Ԫ���������������ﵽ8���û�����ΪNF3��������Nԭ�Ӽ۲���Ӷ���Ϊ3+$\frac{5-1��3}{2}$=4������1�Թµ��Ӷԣ��ռ�ṹΪ�����Σ�����Nԭ�ӵ��ӻ���ʽ�� sp3������F�ĵ縺�Ա�N��N-F�ɼ����Ӷ���Fƫ�ƣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ������NF3�����������γ������ӣ�
�ʴ�Ϊ��N��F�� �����Σ�sp3��F�ĵ縺�Ա�N��N-F�ɼ����Ӷ���Fƫ�ƣ�����NF3��Nԭ�Ӻ˶���¶Ե��ӵ�����������ǿ�������γ���λ������NF3�����������γ������ӣ�
��2��X���⻯��ΪNH3��������ˮ��Ӧ������������ˮ�γ������������ˮ���Ǽ��Է��ӣ��������ܣ��ʰ���������ˮ��
�ʴ�Ϊ��������ˮ��Ӧ������������ˮ�γ������������ˮ���Ǽ��Է��ӣ��������ܣ��ʰ���������ˮ��
��3��ZΪAl����������Ų�ʽ�ǣ�1s22s22p63s23p1������Ԫ��ԭ�ӵ���Χ�����Ų��������ɽ����ڱ��ֳ������������Z����p����
�ʴ�Ϊ��1s22s22p63s23p1��p��
��4���ɾ����ṹ��֪�������к���Ca2+������ĿΪ8��$\frac{1}{8}$+6��$\frac{1}{2}$=4������������Ca2+�����о�����ȫ�����ṹ��֪����֮�����F-������8������Ca2+������λ��Ϊ8�������к���8��F-���ӣ���������Ϊ4��$\frac{78}{{N}_{A}}$g��W��Y�γɵĻ�����ľ����߳�Ϊa pm�������Ϊ��a��10-10cm��3�����ܶ�Ϊ4��$\frac{78}{{N}_{A}}$g�£�a��10-10cm��3=$\frac{4��78}{��a��1{0}^{-10}��^{3}��{N}_{A}}$g•cm-3��
�ʴ�Ϊ��4��8��$\frac{4��78}{��a��1{0}^{-10}��^{3}��{N}_{A}}$��
���� ���⿼��ṹ����λ�ù�ϵӦ�ã��ƶ�Ԫ���ǽ���ؼ�����1���н���NF3�����������γ�������Ϊ�״��㡢�ѵ㣬��4����ע������ʶ����ѧ���������ṹ���ѵ��еȣ�

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�| A�� | ��Һ©��ʱӦ�ر��䲣�����ͻ��� | |
| B�� | ��ʪ��ĵ⻯�ص�����ֽ����Br2��g����NO2 | |
| C�� | ��50mL��Ͳ������0.1000mol•L-1̼������Һ | |
| D�� | ����NH4+ʱ���������м���NaOH��Һ���ȣ���ʪ�����ɫʯ����ֽ�����ݳ������� |

�ش��������⣺
��1���������Ũ���ᷴӦ�IJ���֮һ��TiOSO4����Ӧ�����������ɣ�����Ʒ����������Fe2+��
��2���������������м�����м��Ŀ���Ƿ�ֹFe2+������
��3����ʱ��Һ���к���Fe2+��TiO2+������Mg2+�������ӣ������£����Ӧ���������Ksp���±���ʾ��
| �������� | Fe��OH��2 | TiO��OH��2 | Mg��OH��2 |
| Ksp | 8.0��10-16 | 1.0��10-29 | 1.8��10-11 |
����������Fe2+��TiO2+��Mg2+����Һ��ˮϡ�ͣ���������������ɫ������д���÷�Ӧ�����ӷ���ʽ��TiO2++2H2O�TH2TiO3��+2H+��
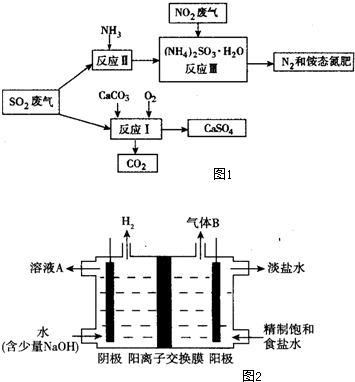
��4��Mg��ԭTiCl4�����б�����1070K���¶��½��У�����Ϊ��Ӧ�ÿ��Ƶķ�Ӧ�����Ǹ���������
��5����800��1000��ʱ���TiO2Ҳ���Ƶú����ѣ�װ����ͼ2��ʾ��ͼ��b�ǵ�Դ�������������ĵ缫��ӦʽTiO2+4e-�TTi+2O2-��
| A�� | Cu$��_{v}^{O_{2}}$CuO$\stackrel{H_{2}SO_{4}}{��}$CuSO4��Һ$\stackrel{�ᾧ}{��}$CuSO4•5H2O | |
| B�� | Al$��_{v}^{O_{2}}$Al2O3$\stackrel{{H}_{2}S{O}_{4}}{��}$Al2��SO4��3��Һ$\stackrel{��������}{��}$Al2��SO4��3 | |
| C�� | FeSO4��Һ$\stackrel{{H}_{2}S}{��}$FeS$��_{����}^{����}$FeS���� | |
| D�� | MgCl2��Һ$��_{����}^{NH_{3}}$Mg��OH��2$\stackrel{����HN{O}_{3}}{��}$Mg��NO3��2��Һ$\stackrel{�ᾧ}{��}$Mg��NO3��•6H2O |
| A�� | ij����Ԫ�شӻ���̬��Ϊ����̬ʱ����Ԫ��һ��������ԭ��Ӧ | |
| B�� | ������Ԫ�ص����ӣ�һ������������ | |
| C�� | ���������ӱ���ԭһ���õ��������� | |
| D�� | ��������ԭ��Ӧ�У���������һ������ԭ |
| A�� | 3.2gͭ������ϡ���ᷴӦ������ת�Ƶ���0.1NA | |
| B�� | 35.5g�������أ�KO2�������������ӵ���ĿΪNA | |
| C�� | ��״���½�0.5molSO2������0.5molH2S�����Ϻ�����ķ�������ΪNA | |
| D�� | 6g���������к�Si-O����Ϊ0.2NA |
 ���ķ�ˮ����ͨ�����������س�ȥ����ԭ����ͼ��ʾ��
���ķ�ˮ����ͨ�����������س�ȥ����ԭ����ͼ��ʾ��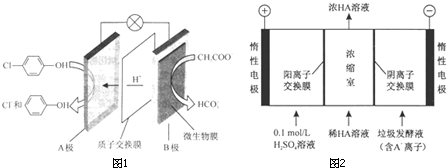
 -OH+2e-+H+�T
-OH+2e-+H+�T -OH+Cl-��
-OH+Cl-��