��Ŀ����
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã�����Ӧ��ѧ����ش��������⣺
��1�����������γɻ������������Ԫ�ص�ԭ�ӽṹʾ��ͼ ��д������ԭ�Ӱ뾶��С��ԭ���γ�10������Ϊ��������ṹ�Ļ�����ĵ���ʽ_________���õ���ʽ��ʾ�ݺ͢��γɻ�����Ĺ���________________________________________��
��2���ܢݢ��γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________�������ӷ��ţ����ۢߢ������������Ӧˮ�����������ǿ������˳��Ϊ____���ѧʽ����
��3���ݺ͢��γɻ�����ľ�������Ϊ__________��
��4����ЩԪ���γɵ��������У�������ˮ����������ǿ��������ǿ�Ӧ����___________���ѧʽ����д������ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽ__________��
��5��X��Y�ɢ٢ڢ��е����ֻ�����Ԫ����ɡ�X����Һ����С�մ�Ӧ����Y����X��������ϵ������ķ��ӣ�����Է�������Ϊ46����X������Ϊ ��д��X��Һ��С�մ�Ӧ�Ļ�ѧ����ʽΪ_______________��
| ������ | IA | | 0 | |||||
| 1 | �� | ��A | ��A | ��A | ��A | ��A | ��A | |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | �� | | �� | �� | |
��2���ܢݢ��γɵļ����Ӱ뾶�ɴ�С��˳��Ϊ_______________�������ӷ��ţ����ۢߢ������������Ӧˮ�����������ǿ������˳��Ϊ____���ѧʽ����
��3���ݺ͢��γɻ�����ľ�������Ϊ__________��
��4����ЩԪ���γɵ��������У�������ˮ����������ǿ��������ǿ�Ӧ����___________���ѧʽ����д������ݵ�����������Ӧˮ�������Ӧ�����ӷ���ʽ__________��
��5��X��Y�ɢ٢ڢ��е����ֻ�����Ԫ����ɡ�X����Һ����С�մ�Ӧ����Y����X��������ϵ������ķ��ӣ�����Է�������Ϊ46����X������Ϊ ��д��X��Һ��С�մ�Ӧ�Ļ�ѧ����ʽΪ_______________��
��1�� ��1�֣���
��1�֣���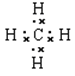 ��1�֣���
��1�֣���
 ��1�֣���
��1�֣���
��2��S2->O2->Na+(1��)��HClO4>HNO3>H2SiO3(1��) ��3�����Ӿ���(1��)
��4��Al2O3(1��) ��Al2O3+2OH����2AlO�� 2+H2O(2��)
��5������(1��)��HCOOH+NaHCO3�� HCOONa +CO2��+H2O(2��)
 ��1�֣���
��1�֣��� ��1�֣���
��1�֣��� ��1�֣���
��1�֣�����2��S2->O2->Na+(1��)��HClO4>HNO3>H2SiO3(1��) ��3�����Ӿ���(1��)
��4��Al2O3(1��) ��Al2O3+2OH����2AlO�� 2+H2O(2��)
��5������(1��)��HCOOH+NaHCO3�� HCOONa +CO2��+H2O(2��)
�������������Ԫ�آ١����ڱ��е�λ�ÿ�֪�����Ƿֱ���H��C��N��O��Na��Al��Si��S��Cl��
��1���γɻ������������Ԫ����̼Ԫ�أ�̼Ԫ�ص�ԭ��������6����ԭ�ӽṹʾ��ͼΪ
 ��ԭ�Ӱ뾶��С������Ԫ�أ��������ԭ�Ӱ뾶��С��ԭ���γ�10������Ϊ��������ṹ�Ļ������Ǽ��飬�����ʽΪ
��ԭ�Ӱ뾶��С������Ԫ�أ��������ԭ�Ӱ뾶��С��ԭ���γ�10������Ϊ��������ṹ�Ļ������Ǽ��飬�����ʽΪ ���ݺ͢��γɻ����������ƣ��������Ӽ������ӻ�������γɹ��̿ɱ�ʾΪ
���ݺ͢��γɻ����������ƣ��������Ӽ������ӻ�������γɹ��̿ɱ�ʾΪ ��
����2���ܢݢ��γɵļ����ӷֱ���O2����Na����S2�������ӵĺ�����Ӳ���Խ�����Ӱ뾶Խ��������Ų���ͬ���������Ӱ뾶��ԭ���������������С�����Ԣܢݢ��γɵļ����Ӱ뾶�ɴ�С��˳����S2->O2->Na+���ǽ�����Խǿ������������Ӧˮ���������Խǿ���ۢߢ�����Ԫ�صķǽ�����ǿ��˳����Cl��N��Si��������������Ӧˮ�����������ǿ������˳��ΪHClO4��HNO3��H2SiO3��
��3���ݺ͢��γɻ�������NaCl���侧������Ϊ���Ӿ��塣
��4��������ˮ����������ǿ��������ǿ�Ӧ����Al2O3���Ƶ�����������Ӧˮ�������������ƣ�����������Ӧ�����ӷ���ʽΪAl2O3+2OH����2AlO�� 2+H2O��
��5��X����Һ����С�մ�Ӧ����Y����˵��X������ǿ��̼��ġ�����ΪX����H��C��OԪ��ɣ����X��������ϵ������ķ��ӣ�����Է�������Ϊ46����Xһ���Ǽ��ᣬ��̼�����ɷ�Ӧ�Ļ�ѧ����ʽΪHCOOH+NaHCO3�� HCOONa +CO2��+H2O��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
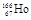 ��ԭ�Ӻ����������������֮����
��ԭ�Ӻ����������������֮����
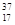 Cl��
Cl��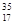 ClΪ��ͬ�ĺ��أ�����˵����ȷ����
ClΪ��ͬ�ĺ��أ�����˵����ȷ����