��Ŀ����
��������һ����Ҫ�����ȼ�ϣ�Ҳ�������������������ȣ��Գ��������ƻ����á���ҵ������ú���������ˮú�����ϳɶ����ѡ�
��ش��������⣺
��1��ú����������Ҫ��ѧ����ʽΪ ��
��2������ˮú���ϳɶ����ѵ��ܷ�Ӧʽ�ɱ�ʾΪ3CO(g)��3H2(g) CH3OCH3(g)��CO2(g) ��H�� 0��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ������ĸ���ţ���
CH3OCH3(g)��CO2(g) ��H�� 0��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ������ĸ���ţ���
��3����֪��Ӧ2CH3OH(g)  CH3OCH3(g)��H2O(g),ij�¶��µ�ƽ�ⳣ��Ϊ400.���¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)��H2O(g),ij�¶��µ�ƽ�ⳣ��Ϊ400.���¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
��ͨ������Ƚϴ�ʱ�����淴Ӧ���ʵĴ�С��
��������CH3OH��10min��Ӧ�ﵽƽ�⣬�Լ����ʱCH3OH�����ʵ���Ũ�Ⱥ�ʱ����CH3OH�Ļ�ѧ��Ӧ���ʣ�
��ش��������⣺
��1��ú����������Ҫ��ѧ����ʽΪ ��
��2������ˮú���ϳɶ����ѵ��ܷ�Ӧʽ�ɱ�ʾΪ3CO(g)��3H2(g)
 CH3OCH3(g)��CO2(g) ��H�� 0��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ������ĸ���ţ���
CH3OCH3(g)��CO2(g) ��H�� 0��һ�������µ��ܱ������У����ܷ�Ӧ�ﵽƽ�⣬Ҫ���CO��ת���ʣ����Բ�ȡ�Ĵ�ʩ�� ������ĸ���ţ���| A�����¸�ѹ | B��������� |
| C������CO2��Ũ�� | D������CO��Ũ�� E������������� |
 CH3OCH3(g)��H2O(g),ij�¶��µ�ƽ�ⳣ��Ϊ400.���¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�
CH3OCH3(g)��H2O(g),ij�¶��µ�ƽ�ⳣ��Ϊ400.���¶��£����ܱ������м���CH3OH����Ӧ��ijʱ�̲�ø���ֵ�Ũ�����£�| ���� | CH3OH | CH3OCH3 | H2O |
| Ũ��/��mol/L�� | 0.44 | 0.6 | 0.6 |
��������CH3OH��10min��Ӧ�ﵽƽ�⣬�Լ����ʱCH3OH�����ʵ���Ũ�Ⱥ�ʱ����CH3OH�Ļ�ѧ��Ӧ���ʣ�
��10�֣�
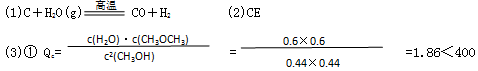
����������У� v(��) �� v���棩
�� 2CH3OH(g) CH3OCH3(g)��H2O(g)
CH3OCH3(g)��H2O(g)
ʼ̬��mol/L�� c0 0 0
ijʱ�� 0.44 0.6 0.6
z��ߵ� 0.44+1.2 0 0 c��CH3OH��=0.44+1.2=1.64mol/L
��ƽ�ⷴӦŨ�� ��c ��c/2 ��c/2
ƽ��Ũ�� 0.44-��c ��c/2 ��c/2
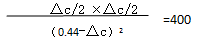
��c ="1.6mol/L " ƽ�⣬ʱCH3OH�����ʵ���Ũ�� 0.04mol/L
��ʱ����CH3OH�Ļ�ѧ��Ӧ����Ϊ0.16mol/��L��min��
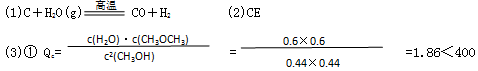
����������У� v(��) �� v���棩
�� 2CH3OH(g)
 CH3OCH3(g)��H2O(g)
CH3OCH3(g)��H2O(g)ʼ̬��mol/L�� c0 0 0
ijʱ�� 0.44 0.6 0.6
z��ߵ� 0.44+1.2 0 0 c��CH3OH��=0.44+1.2=1.64mol/L
��ƽ�ⷴӦŨ�� ��c ��c/2 ��c/2
ƽ��Ũ�� 0.44-��c ��c/2 ��c/2
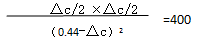
��c ="1.6mol/L " ƽ�⣬ʱCH3OH�����ʵ���Ũ�� 0.04mol/L
��ʱ����CH3OH�Ļ�ѧ��Ӧ����Ϊ0.16mol/��L��min��
��

��ϰ��ϵ�д�
�����Ŀ
 ������Ӧ�ﵽƽ��ʱ��CO���������Ϊx����ά��������������¶Ȳ��䣬��ʼ���ʰ�����������ȳ���������У��ﵽƽ��ʱCO�������������x����(����)
������Ӧ�ﵽƽ��ʱ��CO���������Ϊx����ά��������������¶Ȳ��䣬��ʼ���ʰ�����������ȳ���������У��ﵽƽ��ʱCO�������������x����(����) 2E(g)������ʼʱֻ����2 mol E(g)����ƽ��ʱ��E��ת����Ϊ40%������ʼʱ����2 mol M��1 mol N�Ļ�����壬��ƽ��ʱ��������ѹǿ����ʼʱ������
2E(g)������ʼʱֻ����2 mol E(g)����ƽ��ʱ��E��ת����Ϊ40%������ʼʱ����2 mol M��1 mol N�Ļ�����壬��ƽ��ʱ��������ѹǿ����ʼʱ������ H2(g)��I2(g)����H��0����t1ʱ�ﵽƽ�⣬t2ʱ�������²����£���t3ʱ�ִﵽ��ƽ�⣬������һ�仯��ͼ���� ( )
H2(g)��I2(g)����H��0����t1ʱ�ﵽƽ�⣬t2ʱ�������²����£���t3ʱ�ִﵽ��ƽ�⣬������һ�仯��ͼ���� ( )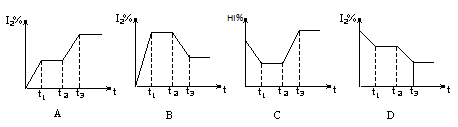
 2C��g������2 s ����C��Ũ��Ϊ0.6 mol/L������˵������ȷ���ǣ���������
2C��g������2 s ����C��Ũ��Ϊ0.6 mol/L������˵������ȷ���ǣ��������� VO2��+ 2H����V2+��
VO2��+ 2H����V2+�� N2(g)+2CO2(g)����H��0��
N2(g)+2CO2(g)����H��0��
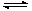 2B(g)+C(g) ����H>0��Ҫʹ�淴Ӧ��������A��Ũ�ȼ�С��ֻ�ı�һ����������Ӧ��ȡ�Ĵ�ʩ�ǣ� ��
2B(g)+C(g) ����H>0��Ҫʹ�淴Ӧ��������A��Ũ�ȼ�С��ֻ�ı�һ����������Ӧ��ȡ�Ĵ�ʩ�ǣ� ��