��Ŀ����
��7�֣�Ϊ���о���������Թ�������ֽ����ʵ�Ӱ�죬ijͬѧ��������ʵ�飺
��ش��������⣺
��1����������ֽ�Ļ�ѧ����ʽΪ �������������� ��
��2��ʵ��ٵ�Ŀ����___________________________________________________��ʵ���еμ�FeCl3��Һ��Ŀ����________________________________________��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������_________________________����ʵ�������ṩ���Լ�����������
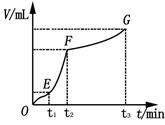
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ�������ж�OE��EF��FG�����У�___________�λ�ѧ��Ӧ������졣
| ��� | ���� | ʵ������ |
| �� | �ֱ����Թ�A��B�м���5 mL 5% H2O2��Һ��������2��1 mol��L��1 FeCl3��Һ�����Թ��о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��н��ݣ����Թ�B����ʢ��40��������ˮ���ձ��н��� | �Թ�A�����������ݲ����� �Թ�B�в��������������� |
| �� | ��ȡ��֧�Թֱܷ����5 mL 5% H2O2��Һ��5 mL 10% H2O2��Һ | �Թ�A��B�о�δ���Լ��������ݲ��� |
��1����������ֽ�Ļ�ѧ����ʽΪ �������������� ��
��2��ʵ��ٵ�Ŀ����___________________________________________________��ʵ���еμ�FeCl3��Һ��Ŀ����________________________________________��
��3��ʵ���δ�۲쵽Ԥ�ڵ�ʵ������Ϊ�˰�����ͬѧ�ﵽʵ��Ŀ�ģ�������Ķ����������ĸĽ������_________________________����ʵ�������ṩ���Լ�����������
��4��ijͬѧ��50 mLһ��Ũ�ȵ�H2O2��Һ�м���һ�����Ķ������̣��ų�������������״���£��뷴Ӧʱ��Ĺ�ϵ����ͼ��ʾ�������ж�OE��EF��FG�����У�___________�λ�ѧ��Ӧ������졣

��1��2H2O2  2H2O+O2����1�֣� ��2���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죨1�֣�
2H2O+O2����1�֣� ��2���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죨1�֣�
��Ϊ�������ӿ�H2O2�ֽ����� ��1�֣�
��3������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol��L��1 FeCl3��Һ�����۲�������ݵ����ʣ�2�֣� ��4��EF��2�֣�
 2H2O+O2����1�֣� ��2���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죨1�֣�
2H2O+O2����1�֣� ��2���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죨1�֣���Ϊ�������ӿ�H2O2�ֽ����� ��1�֣�
��3������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol��L��1 FeCl3��Һ�����۲�������ݵ����ʣ�2�֣� ��4��EF��2�֣�
�����������1����������ֽ⣬���������ڴ˷�Ӧ����������������ã��ܼӿ��������ֽ�������������ʣ��ʴ�Ϊ��2H2O2
 2H2O+O2����
2H2O+O2���� ��2���ֱ����Թ�A��B�м��� 5mL 5% H2O2��Һ��������1��2 ��1mol/L FeCl3��Һ�����Թ��о����������ݳ��֣�˵����������ֽ��ܷ������Թ�A��B�о����������ݳ���ʱ�����Թ�A����ʢ��5��������ˮ���ձ��У����Թ�B����ʢ��40��������ˮ���ձ��У���֧�Թܲ�ͬ�����Թ�A���¶ȱ��Թ�B���¶ȵͣ�˵���о������¶ȶԷ�Ӧ���ʵ�Ӱ�죬����ʼ�ӵμ�FeCl3��Һ��Ŀ�ļӿ�H2O2�ֽ⣬�ʴ�Ϊ���о��¶ȶ�H2O2�ֽ����ʵ�Ӱ�죻�ӿ�H2O2�ֽ����ʣ�ʹʵ���������ڹ۲죻
��3��Ӱ�컯ѧ��Ӧ���ʵ����������Ũ�ȡ��¶ȡ������ѹǿ������������ı��������ȡ��֧�Թֱܷ���� 5mL 5%H2O2��Һ�� 5mL10%H2O2��Һ���Թ�A��B�о�δ�����ݲ�����Ϊ�ӿ췴Ӧ���ʣ��ɴ��¶ȡ��������Ӱ��Ƕȿ��ǣ��ʴ�Ϊ������֧�Թ�ͬʱ����ʢ����ͬ�¶���ˮ���ձ��У�������֧�Թ���ͬʱ����2��1mol/L FeCl3��Һ���۲�������ݵ����ʣ�
��4����ͼ��������ʾʱ�䣬�������ʾ��������������ʱ��Խ�����ɵ�����Խ�࣬��Ӧ����Խ�죬���ߵ�б��Խ����������������EF�Ρ�

��ϰ��ϵ�д�
 �̲�ȫ���ִʾ�ƪϵ�д�
�̲�ȫ���ִʾ�ƪϵ�д�
�����Ŀ
 N2(g)��CO2(g) ��H����373.2 kJ��mol��1���ﵽƽ���Ϊ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ����ȷ��ʩ�ǣ� ��
N2(g)��CO2(g) ��H����373.2 kJ��mol��1���ﵽƽ���Ϊ��߸÷�Ӧ�����ʺ�NO��ת���ʣ���ȡ����ȷ��ʩ�ǣ� �� Sn2��(aq)��Pb(s)����ϵ��c(Pb2��)��c(Sn2��)�仯��ϵ��ͼ��ʾ�������ж���ȷ����(����)
Sn2��(aq)��Pb(s)����ϵ��c(Pb2��)��c(Sn2��)�仯��ϵ��ͼ��ʾ�������ж���ȷ����(����)
 2SO3(g)��H��0��ij�о�С���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬���з�����ȷ����
2SO3(g)��H��0��ij�о�С���о���������������ʱ���ı�ijһ������������Ӧ��Ӱ�죬���з�����ȷ����