��Ŀ����
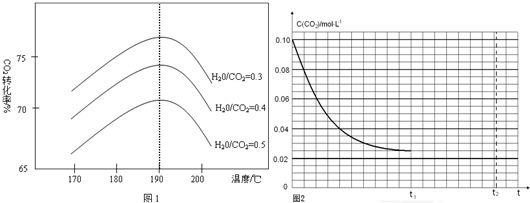
10��������ĸ������ܿ��ø������Ч�ʣ�1m����������ʵ������ˮ��������������������ijЩ������ĸ���Ч�����£�| ���� | MgO | CaO | ZnCl2 | ZnBr2 |
| ������ | 0.008 | 0.2 | 0.8 | 1.1 |
| A�� | MgO �ĸ������ܱ�CaO�� | |
| B�� | ����Ч�ʿ������������������Ӱ뾶��С�й� | |
| C�� | MgCl2�����DZ�CaCl2���õĸ���� | |
| D�� | ����������������ӶԸ������ܵ�Ӱ��������Ӵ� |
���� A��MgO�ĸ���Ч�ʺã�
B��þ���ӱȸ����Ӱ뾶С�������ӱ������Ӱ뾶С�����������Ӻ������ӵİ뾶ԽС����������Խ�ã�
C�����������Ӻ������ӵİ뾶ԽС����������Խ�ã�
D�������ӵĸı�Ը�������Ӱ���
��� �⣺A������Ч���ǣ�1m3������ʵ������ˮ������������MgOΪ0.008��CaOΪ0.2������MgO��1m3������ʵ������ˮ�������������٣�MgO�ĸ���Ч�ʱ�CaO�ã���A����
B�����������Ӻ������ӵİ뾶ԽС����������Խ�ã���B��ȷ��
C��þ���ӱȸ����Ӱ뾶С�������ӱ������Ӱ뾶С��MgCl2��CaCl2�������ܸ��ã���C��ȷ��
D���Աȱ����������ӱ仯�ĸ���Ч����ֵ�������Ӹı����Ч�ʱ仯�������Ӹı����Ч�ʱ仯С����D��ȷ��
��ѡ��A��
���� ���⿼�黯ѧӦ�ã���Ŀ�ѶȲ���������Ϣ�����⣬ͨ���Ķ�����������Ч�ʵĺ��壬ͨ�����е����ʵ���ɺ���Ч�ʹ�ϵ���Ƚϡ�������ý��ۣ�

��ϰ��ϵ�д�
�����Ŀ
20���������ӷ���ʽ����ȷ���ǣ�������
| A�� | ���ռ���Һ��ͨ�������Ķ�����̼ 2OH-+CO2=CO32-+H2O | |
| B�� | ���Ȼ�����Һ�еμӹ���Ũ��ˮ Al3++3OH-�TAl��OH��3�� | |
| C�� | ��ˮ������ͨ������������̼ SiO32-+CO2+H2O=H2SiO3��+CO32- | |
| D�� | ��������������Һ��Ӧ 2Al+2OH-+6H2O�T2AlO2-+3H2�� |
1����пƬ��ͭƬ�õ�����������ϡ������Һ�У���������������ǣ�������
| A�� | пƬ��������п����������Ӧ | B�� | ͭƬ������ | ||
| C�� | ��Һ�е�pHֵ���� | D�� | ��Һ�е�H+���ƶ� |
18�����л�ѧ�����������ȷ���ǣ�������
| A�� | Cl-�Ľṹʾ��ͼ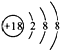 | B�� | MgCl2�ĵ���ʽΪ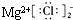 | ||
| C�� | CCl4�Ľṹʽ��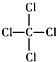 | D�� | ������ķ���ʽΪHClO |
5��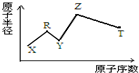 ���ж���������Ԫ��X��Y��Z��R��T��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T�γɵ�Z2T ���������ƻ�ˮ�ĵ���ƽ�⣮����Ԫ�ص�ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ��ͼ��ʾ�������ƶ���ȷ���ǣ�������
���ж���������Ԫ��X��Y��Z��R��T��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T�γɵ�Z2T ���������ƻ�ˮ�ĵ���ƽ�⣮����Ԫ�ص�ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ��ͼ��ʾ�������ƶ���ȷ���ǣ�������
 ���ж���������Ԫ��X��Y��Z��R��T��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T�γɵ�Z2T ���������ƻ�ˮ�ĵ���ƽ�⣮����Ԫ�ص�ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ��ͼ��ʾ�������ƶ���ȷ���ǣ�������
���ж���������Ԫ��X��Y��Z��R��T��Rԭ�������������ǵ��Ӳ�����2����Y��Z���γ�Z2Y��Z2Y2�����ӻ����Z��T�γɵ�Z2T ���������ƻ�ˮ�ĵ���ƽ�⣮����Ԫ�ص�ԭ�Ӱ뾶��ԭ�������Ĺ�ϵ��ͼ��ʾ�������ƶ���ȷ���ǣ�������| A�� | ԭ�Ӱ뾶�����Ӱ뾶�����㣺Y��Z | |
| B�� | �⻯��ķе�����Y��T��R | |
| C�� | ����������Ӧ��ˮ��������ԣ�T��R | |
| D�� | ��X��R��Y��Z����Ԫ����ɵĻ�����ˮ��Һһ���Լ��� |
15��������Һ�У��й����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A�� | ����������Һ�еμ�ϡ����õ���pH=5�Ļ����Һ��c��Na+��=c��NO3-�� | |
| B�� | 1.0 mol•L-1Na2C2O4��Һ��c��OH-��=c��HC2O4-��+c��H+��+c��H2C2O4�� | |
| C�� | pH=8.0��KHS��Һ�У�c��K+����c��HS-����c��OH-����c��S2-����c��H+�� | |
| D�� | ��0.2 mol•L-1��ijһԪ��HA��Һ��0.1 mol•L-1NaOH��Һ�������Ϻ���ҺpH����7����Ӧ��Ļ��Һ�У�c��OH-��+c��A-��=c��H+��+c��HA�� |
19��ij������X���ܱ��������Լ��Ⱦͷֽ�ΪNH3��H2O��CO2��������һʵ����ʵ�ܵó��Ľ����ǣ�������
| A�� | X������Ԫ����� | B�� | X�ǻ����� | C�� | X����ˮ | D�� | X ���ȶ� |
20������ֽ��������������һ����Ҫ��ʵ�鷽�������±���ʾ��ʵ���У��ɼ��ȣ���������ֽ��ѡ�á�����Ӧ���۲���ȷ��һ���ǣ�������
| ѡ�� | �Լ�B | ʪ�����ֽA | ���� | ���� |
| A | ��ˮ | pH��ֽ | �ȱ�����ɫ | ��ˮ�������Ժ�Ư���� |
| B | Ũ��ˮ����ʯ�� | ��ɫʯ����ֽ | ��� | ����Ϊ�������� |
| C | Na2SO3������ | Ʒ����ֽ | ��ɫ | SO2����Ư���� |
| D | MnO2��Ũ���� | ����KI��ֽ | ���� | �����ԣ�Cl2��I2 |
| A�� | A | B�� | B | C�� | C | D�� | D |