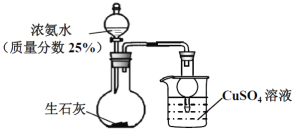
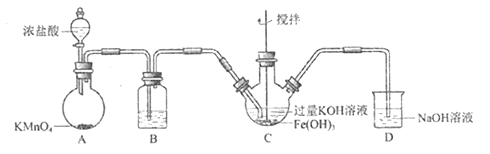
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩ“‘ΙΛ“ΒΖœΧζΚΆΝρΥαοßΈΣ‘≠ΝœΩ…“‘÷Τ»Γ![]() ΚΆΝρΥαΧζοßΘ§Τδ÷Τ±ΗΝς≥ΧΆΦ»γœ¬,«κΗυΨίΧβ“βΆξ≥…œ¬Ν–ΧνΩ’ΘΚ
ΚΆΝρΥαΧζοßΘ§Τδ÷Τ±ΗΝς≥ΧΆΦ»γœ¬,«κΗυΨίΧβ“βΆξ≥…œ¬Ν–ΧνΩ’ΘΚ
![]() ΧΦΥαΡΤ»ή“ΚΡή≥ΐ»ΞθΞάύ”ΆΈέΘ§Τδ‘≠“ρ «
ΧΦΥαΡΤ»ή“ΚΡή≥ΐ»ΞθΞάύ”ΆΈέΘ§Τδ‘≠“ρ «![]() ”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ
”ΟάκΉ”ΖΫ≥Χ Ϋ±μ Ψ![]() ______ΘΜΖ¥”ΠΔώ–η“ΣΦ”»» ΐΖ÷÷”Θ§Τδ‘≠“ρ «______Θ°
______ΘΜΖ¥”ΠΔώ–η“ΣΦ”»» ΐΖ÷÷”Θ§Τδ‘≠“ρ «______Θ°
![]() ΖœΧζ–Φ÷–Κ§―θΜ·ΧζΘ§Έό–η‘Ύ÷Τ±Η«Α≥ΐ»ΞΘ§Τδάμ”… «
ΖœΧζ–Φ÷–Κ§―θΜ·ΧζΘ§Έό–η‘Ύ÷Τ±Η«Α≥ΐ»ΞΘ§Τδάμ”… «![]() ”ΟάκΉ”ΖΫ≥Χ ΫΜΊ¥π
”ΟάκΉ”ΖΫ≥Χ ΫΜΊ¥π![]() ______ΘΜ≈–ΕœΖ¥”ΠΔρΆξ»ΪΖ¥”ΠΒΡœ÷œσ «______Θ°
______ΘΜ≈–ΕœΖ¥”ΠΔρΆξ»ΪΖ¥”ΠΒΡœ÷œσ «______Θ°
![]() œ¬Ν–Έο÷ ÷–Ήν ΚœΒΡ―θΜ·ΦΝX «______Θ°
œ¬Ν–Έο÷ ÷–Ήν ΚœΒΡ―θΜ·ΦΝX «______Θ°
![]()
![]()
![]()
![]()
![]() Φ”»»’τΖΔ»ή“ΚY÷°«ΑΘ§–η»Γ…ΌΝΩΒΡ»ή“Κά¥Φλ―ι
Φ”»»’τΖΔ»ή“ΚY÷°«ΑΘ§–η»Γ…ΌΝΩΒΡ»ή“Κά¥Φλ―ι![]() «Ζώ“―»Ϊ≤Ω±Μ―θΜ·Θ§‘ρΥυΦ” ‘ΦΝΈΣ______ΘΜ≈–ΕœΡήΖώ”ΟΥα–‘
«Ζώ“―»Ϊ≤Ω±Μ―θΜ·Θ§‘ρΥυΦ” ‘ΦΝΈΣ______ΘΜ≈–ΕœΡήΖώ”ΟΥα–‘![]() »ή“Κά¥¥ζΧφΘ§≤ΔΥΒΟςΤδάμ”…______Θ°
»ή“Κά¥¥ζΧφΘ§≤ΔΥΒΟςΤδάμ”…______Θ°
![]() ≤ΌΉς
≤ΌΉς![]() ΒΡΟϊ≥Τ «______ΘΜΦλ―ιΝρΥαΧζοß÷–
ΒΡΟϊ≥Τ «______ΘΜΦλ―ιΝρΥαΧζοß÷–![]() ΒΡΖΫΖ® «______Θ°
ΒΡΖΫΖ® «______Θ°
![]() ≥Τ»Γ
≥Τ»Γ![]() ÷ΤΒΟΒΡΝρΥαΧζοßΨßΧεΘ§ΫΪΤδ»ή”ΎΥ°≈δ÷Τ≥…100mL»ή“ΚΘ§Ζ÷≥…ΝΫΒ»ΖίΘ§œρΤδ÷–“ΜΖί»ή“Κ÷–Φ”»κΉψΝΩNaOH»ή“ΚΘ§Ιΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΚσΒΟΒΫ
÷ΤΒΟΒΡΝρΥαΧζοßΨßΧεΘ§ΫΪΤδ»ή”ΎΥ°≈δ÷Τ≥…100mL»ή“ΚΘ§Ζ÷≥…ΝΫΒ»ΖίΘ§œρΤδ÷–“ΜΖί»ή“Κ÷–Φ”»κΉψΝΩNaOH»ή“ΚΘ§Ιΐ¬ΥΓΔœ¥Β”ΓΔΗ…‘οΚσΒΟΒΫ![]() ≥ΝΒμΘΜœρΝμ“ΜΖί»ή“Κ÷–Φ”»κ
≥ΝΒμΘΜœρΝμ“ΜΖί»ή“Κ÷–Φ”»κ![]() »ή“ΚΘ§«ΓΚΟΆξ»ΪΖ¥”ΠΘ°‘ρΗΟΝρΥαΧζοßΨßΧεΒΡΜ·―ß ΫΈΣ______Θ°
»ή“ΚΘ§«ΓΚΟΆξ»ΪΖ¥”ΠΘ°‘ρΗΟΝρΥαΧζοßΨßΧεΒΡΜ·―ß ΫΈΣ______Θ°
ΓΨ¥πΑΗΓΩ![]() …ΐΈ¬Θ§¥ΌΫχΥ°ΫβΘ§»ή“ΚΦν–‘‘ω«ΩΘ§ ΙΖ¥”Π≥δΖ÷Ϋχ––
…ΐΈ¬Θ§¥ΌΫχΥ°ΫβΘ§»ή“ΚΦν–‘‘ω«ΩΘ§ ΙΖ¥”Π≥δΖ÷Ϋχ–– ![]() ΘΜ Χζ–Φ≤Μ‘Ό»ήΫβΘ§Χζ–Φ±μΟφ≤Μ‘Ό”–Τχ≈ί≤ζ…ζΘΜ
ΘΜ Χζ–Φ≤Μ‘Ό»ήΫβΘ§Χζ–Φ±μΟφ≤Μ‘Ό”–Τχ≈ί≤ζ…ζΘΜ ![]() Χζ«ηΜ·ΦΊ»ή“Κ ≤ΜΡήΘ§“ρΈΣ
Χζ«ηΜ·ΦΊ»ή“Κ ≤ΜΡήΘ§“ρΈΣ![]() ΚΆ
ΚΆ![]() ΨυΡή ΙΥα–‘ΒΡ
ΨυΡή ΙΥα–‘ΒΡ![]() »ή“ΚΆ …Ϊ ά以ΫαΨß »Γ…ΌΝΩ―υΤΖΖ≈»κ ‘Ιή÷–Θ§Φ” ΝΩΥ°»ήΫβΘ§‘ΌΦ”»κNaOH»ή“ΚΦ”»»Θ§‘Ύ ‘ΙήΩΎ”Ο Σ»σΒΡΚλ…Ϊ ·»ο ‘÷ΫΦλ―ιΘ§Ω¥ΒΫ ‘÷Ϋ±δ≥…άΕ…Ϊ
»ή“ΚΆ …Ϊ ά以ΫαΨß »Γ…ΌΝΩ―υΤΖΖ≈»κ ‘Ιή÷–Θ§Φ” ΝΩΥ°»ήΫβΘ§‘ΌΦ”»κNaOH»ή“ΚΦ”»»Θ§‘Ύ ‘ΙήΩΎ”Ο Σ»σΒΡΚλ…Ϊ ·»ο ‘÷ΫΦλ―ιΘ§Ω¥ΒΫ ‘÷Ϋ±δ≥…άΕ…Ϊ ![]() Μρ
Μρ![]()
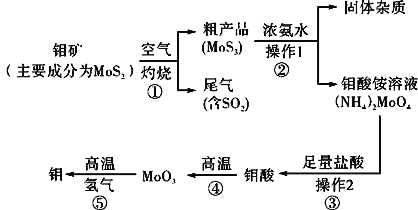
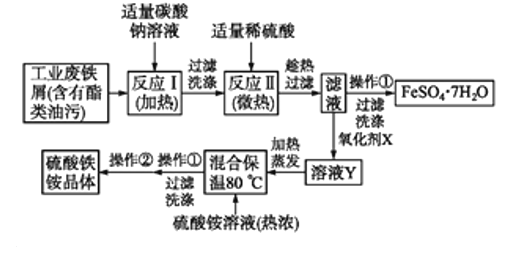
ΓΨΫβΈωΓΩ
ΖœΧζ–Φ÷–Κ§”–θΞάύ”ΆΈέΘ§![]() ΈΣ«ΩΦν»θΥα―ΈΘ§
ΈΣ«ΩΦν»θΥα―ΈΘ§![]() Υ°Ϋβ…ζ≥…
Υ°Ϋβ…ζ≥…![]() ΚΆ
ΚΆ![]() Θ§…ΐΈ¬¥ΌΫχ
Θ§…ΐΈ¬¥ΌΫχ![]() Υ°ΫβΘ§Φν¥ΌΫχ”Ά÷§Υ°ΫβΘ§»ΜΚσΙΐ¬Υœ¥Β”Θ§Φ”»κœΓΝρΥαΘ§ΖΔ…ζΖ¥”ΠIIΈΣ
Υ°ΫβΘ§Φν¥ΌΫχ”Ά÷§Υ°ΫβΘ§»ΜΚσΙΐ¬Υœ¥Β”Θ§Φ”»κœΓΝρΥαΘ§ΖΔ…ζΖ¥”ΠIIΈΣ![]() Θ§≥Ο»»Ιΐ¬ΥΓΔά以ΫαΨßΘ§Ιΐ¬Υœ¥Β”ΒΟΒΫ
Θ§≥Ο»»Ιΐ¬ΥΓΔά以ΫαΨßΘ§Ιΐ¬Υœ¥Β”ΒΟΒΫ![]() ΘΜΝρΥα―«Χζ»ή“ΚΦ”»κΙΐ―θΜ·«β―θΜ·ΈΣΝρΥαΧζ»ή“ΚΘ§Φ”»»’τΖΔ≤ΩΖ÷»ήΦΝΘ§Φ”»κ»»≈®ΒΡΝρΥαοß»ή“ΚΘ§±ΘΈ¬
ΘΜΝρΥα―«Χζ»ή“ΚΦ”»κΙΐ―θΜ·«β―θΜ·ΈΣΝρΥαΧζ»ή“ΚΘ§Φ”»»’τΖΔ≤ΩΖ÷»ήΦΝΘ§Φ”»κ»»≈®ΒΡΝρΥαοß»ή“ΚΘ§±ΘΈ¬![]() Θ§Ω……ζ≥…ΝρΥαΧζοßΘ§Ψ≠ά以ΫαΨßΓΔΙΐ¬Υœ¥Β”Θ§ΉνΚσΨ≠≥ΘΈ¬Νά…ΙΩ…ΒΫ¥ΩΨΜΒΡΝρΥαΧζοßΘ§Ψί¥ΥΖ÷ΈωΫβ¥πΓΘ
Θ§Ω……ζ≥…ΝρΥαΧζοßΘ§Ψ≠ά以ΫαΨßΓΔΙΐ¬Υœ¥Β”Θ§ΉνΚσΨ≠≥ΘΈ¬Νά…ΙΩ…ΒΫ¥ΩΨΜΒΡΝρΥαΧζοßΘ§Ψί¥ΥΖ÷ΈωΫβ¥πΓΘ
![]() ΈΣ«ΩΦν»θΥα―ΈΘ§
ΈΣ«ΩΦν»θΥα―ΈΘ§![]() Υ°Ϋβ…ζ≥…
Υ°Ϋβ…ζ≥…![]() ΚΆ
ΚΆ![]() Θ§Υ°ΫβΖΫ≥Χ ΫΈΣ
Θ§Υ°ΫβΖΫ≥Χ ΫΈΣ![]() Θ§”Ά÷§‘ΎΦν–‘ΧθΦΰœ¬Υ°Ϋβ…ζ≥…Ω…»ή”ΎΥ°ΒΡτ»Υα―ΈΚΆ¥ΦΘΜ―ΈΒΡΥ°ΫβΈΣΈϋ»»Ζ¥”ΠΘ§…ΐΈ¬Θ§¥ΌΫχΥ°ΫβΘ§»ή“ΚΦν–‘‘ω«ΩΘ§ ΙΖ¥”Π≥δΖ÷Ϋχ––ΘΜΙ ¥πΑΗΈΣΘΚ
Θ§”Ά÷§‘ΎΦν–‘ΧθΦΰœ¬Υ°Ϋβ…ζ≥…Ω…»ή”ΎΥ°ΒΡτ»Υα―ΈΚΆ¥ΦΘΜ―ΈΒΡΥ°ΫβΈΣΈϋ»»Ζ¥”ΠΘ§…ΐΈ¬Θ§¥ΌΫχΥ°ΫβΘ§»ή“ΚΦν–‘‘ω«ΩΘ§ ΙΖ¥”Π≥δΖ÷Ϋχ––ΘΜΙ ¥πΑΗΈΣΘΚ![]() ΘΜ…ΐΈ¬Θ§¥ΌΫχΥ°ΫβΘ§»ή“ΚΦν–‘‘ω«ΩΘ§ ΙΖ¥”Π≥δΖ÷Ϋχ––ΘΜ
ΘΜ…ΐΈ¬Θ§¥ΌΫχΥ°ΫβΘ§»ή“ΚΦν–‘‘ω«ΩΘ§ ΙΖ¥”Π≥δΖ÷Ϋχ––ΘΜ
![]() ΖœΧζ–Φ÷–Κ§―θΜ·ΧζΘ§Έό–η‘Ύ÷Τ±Η«Α≥ΐ»ΞΘ§“ρΈΣ―θΜ·ΧζΚΆΥαΖ¥”Π…ζ≥…Χζ―ΈΘ§Χζ―ΈΡή±ΜFeΜΙ‘≠…ζ≥…―«Χζ―ΈΘ§…φΦΑΒΡάκΉ”ΖΫ≥Χ ΫΈΣ
ΖœΧζ–Φ÷–Κ§―θΜ·ΧζΘ§Έό–η‘Ύ÷Τ±Η«Α≥ΐ»ΞΘ§“ρΈΣ―θΜ·ΧζΚΆΥαΖ¥”Π…ζ≥…Χζ―ΈΘ§Χζ―ΈΡή±ΜFeΜΙ‘≠…ζ≥…―«Χζ―ΈΘ§…φΦΑΒΡάκΉ”ΖΫ≥Χ ΫΈΣ![]() ΓΔ
ΓΔ![]() Θ§Χζ–Φ≤Μ‘Ό»ήΫβΘ§Χζ–Φ±μΟφ≤Μ‘Ό”–Τχ≈ί≤ζ…ζΘ§Ζ¥”ΠΔρΆξ»ΪΖ¥”ΠΘΜΙ ¥πΑΗΈΣΘΚ
Θ§Χζ–Φ≤Μ‘Ό»ήΫβΘ§Χζ–Φ±μΟφ≤Μ‘Ό”–Τχ≈ί≤ζ…ζΘ§Ζ¥”ΠΔρΆξ»ΪΖ¥”ΠΘΜΙ ¥πΑΗΈΣΘΚ![]() Θ§
Θ§![]() ΘΜΧζ–Φ≤Μ‘Ό»ήΫβΘ§Χζ–Φ±μΟφ≤Μ‘Ό”–Τχ≈ί≤ζ…ζΘΜ
ΘΜΧζ–Φ≤Μ‘Ό»ήΫβΘ§Χζ–Φ±μΟφ≤Μ‘Ό”–Τχ≈ί≤ζ…ζΘΜ
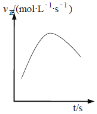
![]() ―θΜ·ΦΝX―θΜ·―«ΧζάκΉ”Θ§Ά§ ±≤Μ“ΐ»κ–¬‘”÷ Θ§Ι ―Γ‘ώΙΐ―θΜ·«βΘΜΙ ¥πΑΗΈΣΘΚbΘΜ
―θΜ·ΦΝX―θΜ·―«ΧζάκΉ”Θ§Ά§ ±≤Μ“ΐ»κ–¬‘”÷ Θ§Ι ―Γ‘ώΙΐ―θΜ·«βΘΜΙ ¥πΑΗΈΣΘΚbΘΜ
![]() ―«ΧζάκΉ””ΟΧζ«ηΜ·ΦΊΦλ―ιΘ§―θΜ·―«Χζ ±Φ”»κΒΡΙΐ―θΜ·«βΙΐΝΩΘ§Ιΐ―θΜ·«β“≤Ρή ΙΥα–‘ΗΏΟΧΥαΦΊΆ …ΪΘ§Ι ≤Μ―ΓΥα–‘ΗΏΟΧΥαΦΊΘΜΙ ¥πΑΗΈΣΘΚΧζ«ηΜ·ΦΊ»ή“ΚΘΜ≤ΜΡήΘ§“ρΈΣ
―«ΧζάκΉ””ΟΧζ«ηΜ·ΦΊΦλ―ιΘ§―θΜ·―«Χζ ±Φ”»κΒΡΙΐ―θΜ·«βΙΐΝΩΘ§Ιΐ―θΜ·«β“≤Ρή ΙΥα–‘ΗΏΟΧΥαΦΊΆ …ΪΘ§Ι ≤Μ―ΓΥα–‘ΗΏΟΧΥαΦΊΘΜΙ ¥πΑΗΈΣΘΚΧζ«ηΜ·ΦΊ»ή“ΚΘΜ≤ΜΡήΘ§“ρΈΣ![]() ΚΆ
ΚΆ![]() ΨυΡή ΙΥα–‘ΒΡ
ΨυΡή ΙΥα–‘ΒΡ![]() »ή“ΚΆ …ΪΘΜ
»ή“ΚΆ …ΪΘΜ
![]() ΝρΥα―«Χζ»ή“Κ≥Ο»»Ιΐ¬ΥΚσΨ≠ά以ΫαΨßΓΔΙΐ¬Υœ¥Β”Θ§ΒΟΒΫ¥ΩΨΜΒΡΝρΥα―«ΧζΨßΧεΘ§≤ΌΉς
ΝρΥα―«Χζ»ή“Κ≥Ο»»Ιΐ¬ΥΚσΨ≠ά以ΫαΨßΓΔΙΐ¬Υœ¥Β”Θ§ΒΟΒΫ¥ΩΨΜΒΡΝρΥα―«ΧζΨßΧεΘ§≤ΌΉς![]() ά以ΫαΨßΘΜΦλ―ιοßΗυάκΉ”ΒΡΖΫΖ®ΈΣΘΚ»Γ…ΌΝΩ―υΤΖΖ≈»κ ‘Ιή÷–Θ§Φ” ΝΩΥ°»ήΫβΘ§‘ΌΦ”»κNaOH»ή“ΚΦ”»»Θ§‘Ύ ‘ΙήΩΎ”Ο Σ»σΒΡΚλ…Ϊ ·»ο ‘÷ΫΦλ―ιΘ§Ω¥ΒΫ ‘÷Ϋ±δ≥…άΕ…ΪΘΜΙ ¥πΑΗΈΣΘΚά以ΫαΨßΘΜ»Γ…ΌΝΩ―υΤΖΖ≈»κ ‘Ιή÷–Θ§Φ” ΝΩΥ°»ήΫβΘ§‘ΌΦ”»κNaOH»ή“ΚΦ”»»Θ§‘Ύ ‘ΙήΩΎ”Ο Σ»σΒΡΚλ…Ϊ ·»ο ‘÷ΫΦλ―ιΘ§Ω¥ΒΫ ‘÷Ϋ±δ≥…άΕ…ΪΘΜ
ά以ΫαΨßΘΜΦλ―ιοßΗυάκΉ”ΒΡΖΫΖ®ΈΣΘΚ»Γ…ΌΝΩ―υΤΖΖ≈»κ ‘Ιή÷–Θ§Φ” ΝΩΥ°»ήΫβΘ§‘ΌΦ”»κNaOH»ή“ΚΦ”»»Θ§‘Ύ ‘ΙήΩΎ”Ο Σ»σΒΡΚλ…Ϊ ·»ο ‘÷ΫΦλ―ιΘ§Ω¥ΒΫ ‘÷Ϋ±δ≥…άΕ…ΪΘΜΙ ¥πΑΗΈΣΘΚά以ΫαΨßΘΜ»Γ…ΌΝΩ―υΤΖΖ≈»κ ‘Ιή÷–Θ§Φ” ΝΩΥ°»ήΫβΘ§‘ΌΦ”»κNaOH»ή“ΚΦ”»»Θ§‘Ύ ‘ΙήΩΎ”Ο Σ»σΒΡΚλ…Ϊ ·»ο ‘÷ΫΦλ―ιΘ§Ω¥ΒΫ ‘÷Ϋ±δ≥…άΕ…ΪΘΜ
![]() ≥Τ»Γ
≥Τ»Γ![]() ―υΤΖΘ§ΫΪΤδ»ή”ΎΥ°≈δ÷Τ≥…100mL»ή“ΚΘ§Ζ÷≥…ΝΫΒ»ΖίΘ§œρΤδ÷–“ΜΖί÷–Φ”»κΉψΝΩNaOH»ή“ΚΘ§Ιΐ¬Υœ¥Β”ΒΟΒΫ
―υΤΖΘ§ΫΪΤδ»ή”ΎΥ°≈δ÷Τ≥…100mL»ή“ΚΘ§Ζ÷≥…ΝΫΒ»ΖίΘ§œρΤδ÷–“ΜΖί÷–Φ”»κΉψΝΩNaOH»ή“ΚΘ§Ιΐ¬Υœ¥Β”ΒΟΒΫ![]() ≥ΝΒμΘ§”ΠΈΣ
≥ΝΒμΘ§”ΠΈΣ![]() Θ§
Θ§![]() Θ§œρΝμ“ΜΖί»ή“Κ÷–Φ”»κ
Θ§œρΝμ“ΜΖί»ή“Κ÷–Φ”»κ![]() »ή“ΚΘ§«ΓΚΟΆξ»ΪΖ¥”ΠΘ§‘ρ
»ή“ΚΘ§«ΓΚΟΆξ»ΪΖ¥”ΠΘ§‘ρ![]() Θ§Υυ“‘
Θ§Υυ“‘![]() ―υΤΖ÷–Κ§”–
―υΤΖ÷–Κ§”–![]() Θ§
Θ§![]() ΈΣ
ΈΣ![]() Θ§‘ρ
Θ§‘ρ![]() ΈΣ
ΈΣ![]() Θ§‘ρ
Θ§‘ρ![]() Θ§
Θ§![]() Θ§
Θ§![]() ΘΚ
ΘΚ![]() ΘΚ
ΘΚ![]() ΘΚ
ΘΚ![]() ΘΚ
ΘΚ![]() ΘΚ2ΘΚ2Θ§Υυ“‘Μ·―ß ΫΈΣ
ΘΚ2ΘΚ2Θ§Υυ“‘Μ·―ß ΫΈΣ![]() Μρ
Μρ![]() ΘΜΙ ¥πΑΗΈΣΘΚ
ΘΜΙ ¥πΑΗΈΣΘΚ![]() Μρ
Μρ![]() ΓΘ
ΓΘ