��Ŀ����
��ο�����ͼ������֪E1��134 kJ��mol��1��E2��368 kJ��mol��1������Ҫ��ش����⣺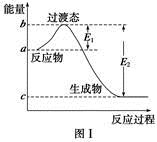

(1)ͼ����1 mol NO2(g)��1 mol CO(g)��Ӧ����CO2��NO�����е������仯ʾ��ͼ�����ڷ�Ӧ��ϵ�м����������Ӧ��������E1�ı仯�� (���������С�����䡱����ͬ)����H�ı仯�� ����д��NO2��CO��Ӧ���Ȼ�ѧ����ʽ�� ��
(2)�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧ���Ȼ�ѧ����ʽ���£�
��CH3OH(g)��H2O(g)===CO2(g)��3H2(g) ��H����49.0 kJ��mol��1
��CH3OH(g)��O2(g)===CO2(g)��2H2(g) ��H����192.9 kJ��mol��1
��֪��H2O(g)===H2O(l) ��H����44 kJ��mol��1����״�����ȼ��ΪҺ̬ˮ���Ȼ�ѧ����ʽΪ ��
(3)�����ʾ�Dz��ֻ�ѧ���ļ��ܲ�����
| ��ѧ�� | P��P | P��O | O===O | P===O |
| ����/kJ��mol��1 | a | b | c | x |
��֪����ȼ����Ϊd kJ��mol��1����������ȫȼ�յIJ���Ľṹ��ͼ����ʾ�������x�� kJ��mol��1(�ú�a��b��c��d�Ĵ�����ʽ��ʾ)��
(1)��С ���� NO2(g)��CO(g)===CO2(g)��NO(g) ��H����234 kJ��mol��1
(2)CH3OH(g)��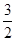 O2(g)===CO2(g)��2H2O(l) ��H����764.7 kJ��mol��1
O2(g)===CO2(g)��2H2O(l) ��H����764.7 kJ��mol��1
(3)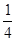 (d��6a��5c��12b)
(d��6a��5c��12b)
����

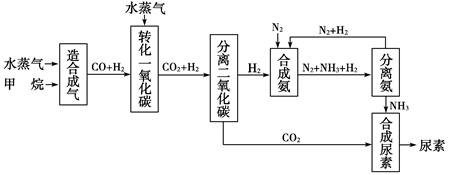
 ��Ȥ������ҵ���ϿƼ�������ϵ�д�
��Ȥ������ҵ���ϿƼ�������ϵ�д���I�����������ѳ�Ϊ��ǰ��δ����һ��ȫ���Կ��⡣Ϊ���Ŀǰȼ��ʹ�ù����еĻ�����Ⱦ���⣬��������ԴΣ�����е�ר���������̫���ܴ�ʹȼ��ѭ��ʹ�õĹ��룬��ͼ��ʾ��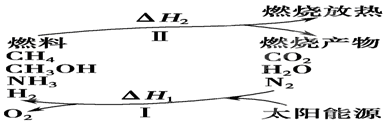
��ش��������⣺
(1)���̢������ת����ʽΪ________��ת��Ϊ________�ܡ�
(2)����ת�������У���H1�ͦ�H2�Ĺ�ϵ��________��
(3)����1 mol��ѧ��������������±���
| ���ۼ� | H��N | H��O | N��N | O��O |
| ����1 mol��ѧ����������/(kJ��mol��1) | 393 | 460 | 941 | 499 |
(II)��һ�Թ��м���0.01mol/L��KMnO4������Һ��0.1mol/LH2C2O4��Һ���ں����·������·�Ӧ��
2KMnO4+5 H2C2O4+3H2SO4��K2SO4+2MnSO4+10CO2+8H2O��5���Ӻ���Mn2+��Ũ��Ϊ0.004mol/L��
��4���Լ���0��5�����ڣ���(H2C2O4)��____________��
(5)�����Ӧ�ӿ�ʼ����һ��ʱ������ʡ�ʱ��ͼ����ͼ��
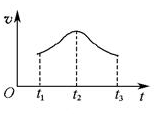 ���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________��
���Խ���t1��t2��t2��t3���ʱ仯��ԭ��______________________________________________________�� ��ͼ�Ǻ���P��s����Cl2��Ӧ���� ��ͼ�е�
��ͼ�е�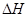 ��ʾ����1mol��������ݣ���������ͼ�ش��������⣺
��ʾ����1mol��������ݣ���������ͼ�ش��������⣺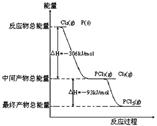
��1��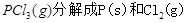 ���Ȼ�ѧ����ʽΪ ��
���Ȼ�ѧ����ʽΪ ��
��2��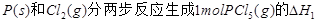 = KJ��mol-1
= KJ��mol-1
��3���о���������ѧ��Ӧ�������仯����H���뷴Ӧ���������ļ����йء����ܿ��Լ�����Ϊ�Ͽ�1mol��ѧ��ʱ�������յ���������1�����Dz��ֻ�ѧ���ļ������ݡ�
��1���ֻ�ѧ���ļ�������
| ��ѧ�� | P-P | P-O | O=O | P=O |
| ����/��kJ��mol-1�� | 198 | 360 | 498 | x |
��֪1mol���ף��ṹ����ͼ��ʾ������ʽΪP4����ȫȼ������P4O10���ṹ����ͼ���ų�2982KJ����������У�x= ��
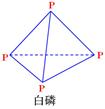 ��P4O10��
��P4O10��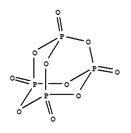
��ѧ��Ӧԭ�����ڹ�ҵ�����Ϳ�������Ҫ����
I������������ѧ��Ӧ��ƽ�ⳣ����K1��K2��K3�����¶ȵĹ�ϵ�ֱ����±���ʾ��
| ��ѧ��Ӧ | ƽ�ⳣ�� | �¶� | |
| 973 K | 1173 K | ||
��Fe(s)��CO2(g) FeO(s)��CO(g) FeO(s)��CO(g) | K1 | 1��47 | 2��15 |
��Fe(s)��H2O(g) FeO(s)��H2(g) FeO(s)��H2(g) | K2 | 2��38 | 1��67 |
��CO(g) ��H2O(g) CO2(g) ��H2(g) CO2(g) ��H2(g) | K3 | �� | �� |
��ش�
��1����Ӧ���� ������ȡ����ȡ�����Ӧ��
��2���ݷ�Ӧ����ڿ��Ƶ���K1��K2��K3֮��Ĺ�ϵ����K3��__________(��K1��K2��ʾ)��
��3��Ҫʹ��Ӧ����һ�������½�����ƽ�����淴Ӧ�����ƶ����ɲ�ȡ�Ĵ�ʩ�� _____(��д��ĸ���)��
A����С��Ӧ�������ݻ�
B������Ӧ�������ݻ�
C�������¶�
D��ʹ�ú��ʵĴ���
E���跨��Сƽ����ϵ�е�CO��Ũ��
��4������Ӧ�۵��淴Ӧ������ʱ��Ĺ�ϵ��ͼ��ʾ��
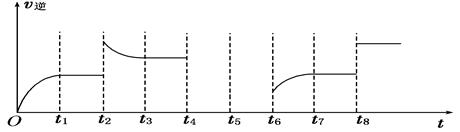
�ٿɼ���Ӧ��t1��t3��t7ʱ���ﵽ��ƽ�⣬��t2��t8ʱ���ı���һ�����������жϸı����ʲô������t2ʱ__________________�� t8ʱ__________________��
����t4ʱ��ѹ�� t6ʱ����Ӧ���Ũ�ȣ�����ͼ�л���t4��t6ʱ�淴Ӧ������ʱ��Ĺ�ϵ�ߡ�
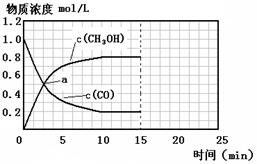
II����5�������˺���������̬ϵͳ�У�����Ҫ�����ȥ��CO2����Ҫ���ṩ�����O2��ij�ֵ绯ѧװ�ÿ�ʵ������ת����2CO2��2CO+O2��CO������ȼ�ϣ���֪��װ�õ�������ӦΪ��4OH--4e-��O2��+2H2O����������ӦΪ ��
��6��ij�ռ�վ����ת��ϵͳ�ľֲ���ͼ��ʾ�����е�ȼ�ϵ�ز���KOH��Һ�����Һ��
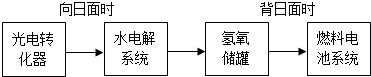
���ij��ʱ���ڣ����������й��ռ���33��6L���壨������ɱ�״��������ö�ʱ����ˮ���ϵͳ��ת�Ƶ��ӵ����ʵ���Ϊ mol��
��CO2Ϊ̼Դ��ȡ��̼�л����Ϊ�����о����㣬����ΪCO2������ȡ��̼��������ѧ���ݣ�
��Ӧ�� CO2(g)+3H2(g) CH3OH(g)+H2O(g) ?H = ��49��0 kJ��mol-1
CH3OH(g)+H2O(g) ?H = ��49��0 kJ��mol-1
��Ӧ��2CO2(g)+6H2(g) CH3CH2OH(g)+3H2O(g) ?H = ��173��6 kJ��mol-1
CH3CH2OH(g)+3H2O(g) ?H = ��173��6 kJ��mol-1
��1��д����CH3OH(g)�ϳ�CH3CH2OH(g)���Ȼ�ѧ��Ӧ����ʽ��
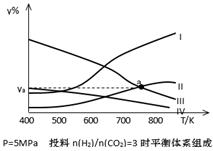
��2���Է�Ӧ����һ���¶��·�Ӧ�ﵽƽ��ı�־�� ��ѡ����)
a����Ӧ�ﲻ��ת��Ϊ������ b��ƽ�ⳣ��K��������
c��CO2��ת���ʲ������� d����������ƽ����Է����������ٸı䡡
��3�����ܱ������У���Ӧ����һ�������ﵽƽ������������㶨�������CO2ת���ʵĴ�ʩ�� ��ѡ���ţ�
| A�������¶� | B������CO2 | C��������� | D����ȥ�״� |
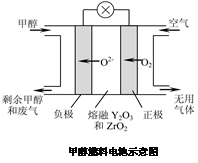
(5)һ���Լ״���ȼ�ϵĵ��ʾ��ͼ��ͼ��д���õ�طŵ�ʱ�����ĵ缫��Ӧʽ�� ��
��֪��Ӧ��Fe(s)+CO2(g) FeO(s)+CO(g)����H=akJ��mol-1,ƽ�ⳣ��ΪK;
FeO(s)+CO(g)����H=akJ��mol-1,ƽ�ⳣ��ΪK;
��Ӧ��CO(g)+1/2O2(g) CO2(g)����H=bkJ��mol-1;
CO2(g)����H=bkJ��mol-1;
��Ӧ��Fe2O3(s)+3CO(g) 2Fe(s)+3CO2(g)����H=ckJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
2Fe(s)+3CO2(g)����H=ckJ��mol-1������ڲ�ͬ�¶���,Kֵ����:
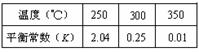
| �¶�/�� | 500 | 700 | 900 |
| K | 1.00 | 1.47 | 2.40 |
(1)��500 ��ʱ���з�Ӧ��,CO2����ʼŨ��Ϊ2 mol��L-1,CO��ƽ��Ũ��Ϊ����������
(2)��Ӧ��Ϊ��������(ѡ����ȡ����ȡ�)��Ӧ��
(3)700 ��ʱ��Ӧ�ٴﵽƽ��״̬,Ҫʹ��ƽ�������ƶ�,������������ʱ,���Բ�ȡ�Ĵ�ʩ����������(�����)��
A.��С��Ӧ����� B.ͨ��CO2
C.�¶����ߵ�900 �� D.ʹ�ú��ʵĴ���
E.����Fe����
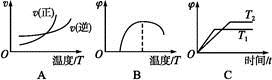
(4)����ͼ����Ϸ�Ӧ�ٵ�����������(�����)(ͼ��vΪ����,��Ϊ�������CO����,TΪ�¶���T1>T2)��
(5)�ɷ�Ӧ�ٺ͢ڿ����,��Ӧ2Fe(s)+O2(g)
 2FeO(s)�Ħ�H=����������
2FeO(s)�Ħ�H=���������� (6)�����ø�˹����д��Fe(����)��O2(����)�����õ�Fe2O3(����)���Ȼ�ѧ����ʽ:�� ��
�Ҵ����ͺ�������35%,ʹȼ��ȼ�ո��ӳ��,ʹ�ó����Ҵ����ͣ�β���ŷŵ�CO��̼�⻯����ƽ������30%����,��Ч�Ľ��ͺͼ������к���β���ŷš���������ʹ���Ҵ����Ͳ����ܼ���NOx���ŷţ���NOx����Ч������Ϊ�����������Ҫ���⡣NOx��������У��γ����꣬��ɿ�����Ⱦ��NOx����һ�ֺ���ɫ���壬������ˮ�ķ���ʽ�� ��
��2����֪NO2��N2O4�Ľṹʽ�ֱ���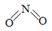 ��
��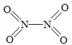 ��
��
| ���� | NO2 | N2O4 | |
| ��ѧ�� | N��O | N��N | N��O |
| ���ܣ�kJ/mol�� | 466 | 167 | 438 |
д��NO2ת��N2O4���Ȼ�ѧ����ʽ ��
��3���о���Ա������β��ϵͳ��װ�ô�ת����������Ч����NOx���ŷš�
�� д����CO��ԭNO����N2�Ļ�ѧ����ʽ ��
�� ��ʵ������ģ�´˷�Ӧ����һ�������µ��ܱ������У����NOת��ΪN2��ת�������¶ȱ仯�����n (NO)/n(CO)�����仯�������ͼ��
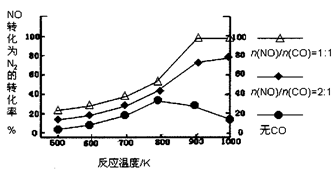
Ϊ�ﵽNOת��ΪN2�����ת���ʣ�Ӧ��ѡ�õ��¶Ⱥ�n(NO)/n(CO)�����ֱ�Ϊ �� ���÷�Ӧ��?H 0�����������������������
��4���� CxHy(��)����ԭNOxҲ����������������������Ⱦ�����ʡ�CH4��NO������Ӧ�Ļ�ѧ����ʽΪ ��
 CH3OH(g) ��H1����90.7 kJ��mol��1
CH3OH(g) ��H1����90.7 kJ��mol��1 CH3OCH3(g)��CO2(g)�ġ�H�� kJ��mol-1��
CH3OCH3(g)��CO2(g)�ġ�H�� kJ��mol-1��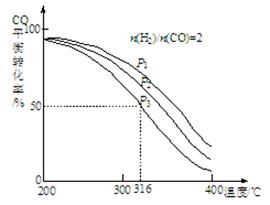
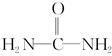 ����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��
����д�����ֺ���̼��˫�������ص�ͬ���칹��Ľṹ��ʽ��