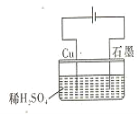
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩΡ≥ΩΈΆβ–Υ»Λ–ΓΉι”Ο0.1000 molΓΛL1ΒΡNaOH±ξΉΦ»ή“ΚΒΈΕ®Έ¥÷Σ≈®Ε»ΒΡ―ΈΥα»ή“ΚΘ§ Β―ι≤ΌΉς»γœ¬Θ§«κΆξ≥…“‘œ¬Έ ΧβΓΘ
AΘ°___________________________ΓΘ
BΘ°Ζ÷±π”Ο’τΝσΥ°œ¥Η…ΨΜΥα ΫΒΈΕ®ΙήΚΆΦν ΫΒΈΕ®ΙήΓΘ
CΘ°”Ο¥ΐ≤βΕ®ΒΡ―ΈΥα»ή“Κ»σœ¥Υα ΫΒΈΕ®ΙήΓΘ
DΘ°”ΟΥα ΫΒΈΕ®Ιή»ΓœΓ―ΈΥα 25.00 mLΘ§ΉΔ»κ ¬œ»œ¥Η…ΨΜΒΡΉΕ–ΈΤΩ÷–Θ§Φ”»κ÷Η ΨΦΝΓΘ
EΘ°Φν ΫΒΈΕ®Ιή”Ο±ξΉΦΒΡNaOH»ή“Κ»σœ¥ΚσΘ§ΫΪ±ξΉΦ“ΚΉΔ»κΦν ΫΒΈΕ®ΙήΩΧΕ»ΓΑ0Γ±“‘…œ2ΓΪ3 cm¥ΠΘ§‘ΌΑ―Φν ΫΒΈΕ®ΙήΙΧΕ®ΚΟΘ§≈≈ΨΓΦβΉλ≤ΩΖ÷ΒΡΤχ≈ίΘ§≤ΔΒςΫΎ“ΚΟφ÷ΝΩΧΕ»ΓΑ0Γ±ΜρΓΑ0Γ±ΩΧΕ»“‘œ¬ΓΘ
FΘ°Α―ΉΕ–ΈΤΩΖ≈‘ΎΒΈΕ®Ιήœ¬ΟφΘ§ΤΩœ¬Βφ“Μ’≈ΑΉ÷ΫΘ§±ΏΒΈ±Ώ“ΓΕ·ΉΕ–ΈΤΩ÷±÷ΝΒΈΕ®÷’ΒψΘ§Φ«œ¬ΒΈΕ®Ιή“ΚΟφΥυ‘ΎΩΧΕ»ΓΘ
GΘ°Νμ»ΓΉΕ–ΈΤΩΘ§‘Ό÷ΊΗ¥≤ΌΉς“Μ¥ΈΓΘ
Θ®1Θ©Ε®ΒΈΙή‘Ύ Ι”Ο«Α–ηΫχ––ΒΡ≤ΌΉςA «___________________________ΓΘ
Θ®2Θ©ΒΈΕ® Β―ιΥυ–ηΒΡ≤ΘΝß“«Τς”–______________ΓΘΘ®ΧνΉ÷ΡΗΘ©
AΘ°Υα ΫΒΈΕ®Ιή BΘ°Φν ΫΒΈΕ®Ιή CΘ°ΝΩΆ≤ DΘ°ΉΕ–ΈΤΩ
EΘ°ΧζΦήΧ® FΘ°ΒΈΕ®ΙήΦ– GΘ°…’±≠ HΘ°ΑΉ÷Ϋ
Θ®3Θ©ΗΟ–ΓΉιΆ§―ß―Γ”ΟΖ”ΧΣΉω÷Η ΨΦΝΘ§ΒΈΕ®÷’ΒψΒΡœ÷œσΈΣ________________________________ΓΘ
Θ®4Θ©ΗΟ–ΓΉιΡ≥“Μ¥ΈΒΈΕ®≤ΌΉς÷–Θ§Υα ΫΒΈΕ®ΙήΒΡ Φ÷’“ΚΟφ»γΆΦΥυ ΨΘ§
‘ρ±Ψ¥ΈΒΈ»κΒΡ―ΈΥαΧεΜΐΈΣ ___________ mLΓΘ
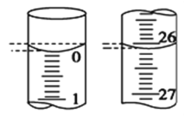
Θ®5Θ©ΗΟ–ΓΉι―ß…ζΡ≥3¥Έ Β―ιΒΡ”–ΙΊ ΐΨίΖ÷±πΦ«¬Φ»γœ¬±μΘΚ
ΒΈΕ®¥Έ ΐ | ¥ΐ≤βHCl»ή“ΚΒΡΧεΜΐ/mL | 0.1000 mol/LNaOHΒΡΧεΜΐΘ®mLΘ© | |
ΒΈΕ®«ΑΩΧΕ» | ΒΈΕ®ΚσΩΧΕ» | ||
ΒΎ“Μ¥Έ | 25.00 | 2.00 | 27.91 |
ΒΎΕΰ¥Έ | 25.00 | 1.56 | 30.30 |
ΒΎ»ΐ¥Έ | 25.00 | 0.22 | 26.31 |
“άΨί…œ±μ ΐΨίΝ– ΫΦΤΥψΗΟHCl»ή“ΚΒΡΈο÷ ΒΡΝΩ≈®Ε»ΈΣ_______________ΓΘ
Θ®6Θ©œ¬Ν–≤ΌΉς÷–Ω…Ρή Ι≤βΕ®ΫαΙϊΤΪΒΆΒΡ «___________(ΧνΉ÷ΡΗ)ΓΘ
AΘ°Υα ΫΒΈΕ®ΙήΈ¥»σœ¥ΨΆ÷±Ϋ”ΉΔ»κ¥ΐ≤β“ΚHCl»ή“Κ
BΘ°ΒΈΕ®«Α ΔΖ≈HCl»ή“ΚΒΡΉΕ–ΈΤΩ”Ο’τΝσΥ°œ¥ΨΜΚσΟΜ”–Η…‘ο
CΘ°Φν ΫΒΈΕ®ΙήΦβΉλ≤ΩΖ÷‘ΎΒΈΕ®«Α”–Τχ≈ίΘ§ΒΈΕ®ΚσΤχ≈ίœϊ ß
DΘ°ΕΝ»ΓNaOH±ξΉΦ“Κ ±Θ§ΩΣ Φ―ω ”ΕΝ ΐΘ§ΒΈΕ®Ϋα χ ±Η© ”ΕΝ ΐ
ΓΨ¥πΑΗΓΩΦλ≤ιΒΈΕ®Ιή «Ζώ¬©Υ° ABDG Β±ΉνΚσ“ΜΒΈ±ξΉΦ“ΚΒΈ»κΘ§»ή“Κ”…Έό…Ϊ±δΈΣ«≥Κλ…Ϊ«“ΑκΖ÷÷”ΡΎ≤ΜΜ÷Η¥ 26.10 0.1040 mol/L AD
ΓΨΫβΈωΓΩ
(1)ΒΈΕ®Ιή Ι”Ο«Α“ΣΦλ≤ι «Ζώ¬©Υ°ΘΜ
(2)ΗυΨί≤ΌΉς≤Ϋ÷η―Γ‘ώ“«ΤςΘΜ
(3)Ζ”ΧΣΒΡ±δ…ΪΖΕΈßΈΣΘΚpH÷ΒΈΣ8~10Θ§–Γ”Ύ8ΈΣΈό…ΪΘ§¥σ”Ύ10ΈΣΚλ…ΪΘΜ
(4)ΒΈΕ®«ΑΕΝ ΐΈΣ0.00mLΘ§ΒΈΕ®ΚσΕΝ ΐΈΣ26.10mLΘΜ
(5)ΗυΨί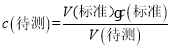 Ϋχ––ΦΤΥψΘΜ
Ϋχ––ΦΤΥψΘΜ
(6)ΗυΨί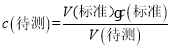 Ϋχ––Έσ≤νΖ÷ΈωΘΜ
Ϋχ––Έσ≤νΖ÷ΈωΘΜ
(1)ΒΈΕ®Ιή Ι”Ο«Α“ΣΦλ≤ι «Ζώ¬©Υ°Θ§Ι ¥πΑΗΈΣΘΚΦλ≤ιΒΈΕ®Ιή «Ζώ¬©Υ°ΘΜ
(2)¥ΐ≤β“ΚΈΣ―ΈΥαΒΈΕ®«Α–η“Σ ΔΖ≈‘Ύ…’±≠÷–Θ§“Τ“Κ ±–η“ΣΥα ΫΒΈΕ®ΙήΘ§ΒΈΕ® ±–η“ΣΉΕ–ΈΤΩ ΔΖ≈Θ§±ξΉΦ“ΚΈΣ«β―θΜ·ΡΤ»ή“ΚΘ§–η“ΣΦν ΫΒΈΕ®ΙήΘ§Ι ¥πΑΗΈΣΘΚABDGΘΜ
(3)Ζ”ΧΣ‘ΎpH÷ΒΈΣ8~10 ±ΈΣΒ≠Κλ…ΪΘ§¥σ”Ύ10 ±ΈΣΚλ…ΪΘ§±ξΉΦ“ΚΈΣ«β―θΜ·ΡΤΘ§¥ΐ≤β“ΚΈΣ―ΈΥαΘ§Φ”»κΖ”ΧΣΚσ≤Μ±δ…ΪΘ§Υφ«β―θΜ·ΡΤΒΡΒΈ»κ»ή“ΚΥα–‘÷πΫΞΦθ»θΘ§ΒΈΕ®÷’Βψ»ή“Κœ‘÷––‘Θ§Ι ¥πΑΗΈΣΘΚΒ±ΉνΚσ“ΜΒΈ±ξΉΦ“ΚΒΈ»κΘ§»ή“Κ”…Έό…Ϊ±δΈΣ«≥Κλ…Ϊ«“ΑκΖ÷÷”ΡΎ≤ΜΜ÷Η¥ΘΜ
(4)ΒΈΕ®«ΑΕΝ ΐΈΣ0.00mLΘ§ΒΈΕ®ΚσΕΝ ΐΈΣ26.10mLΘ§‘ρ±Ψ¥Έ Ι”ΟΒΡ―ΈΥαΧεΜΐΈΣ26.10mLΘ§Ι ¥πΑΗΈΣΘΚ26.10ΘΜ
(5)ΒΎ“Μ¥ΈΥυ”Ο«β―θΜ·ΡΤ»ή“ΚΧεΜΐΈΣ27.91mL-2.00mL=25.91mLΘΜΒΎΕΰ¥ΈΥυ”Ο«β―θΜ·ΡΤ»ή“ΚΧεΜΐΈΣ30.30mL-1.56mL=28.74mLΘΜΒΎΕΰ¥ΈΥυ”Ο«β―θΜ·ΡΤ»ή“ΚΧεΜΐΈΣ26.31mL-0.22mL=26.09mLΘΜΒΎΕΰ¥ΈΤΪ≤νΫœ¥σ…α»ΞΘ§‘ρ±Ψ¥ΈΒΈΕ®Υυ”Ο«β―θΜ·ΡΤ»ή“ΚΧεΜΐΈΣΘΚ26.00mLΘ§ 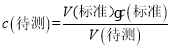 =
=![]() ΘΜΙ ¥πΑΗΈΣΘΚ0.1040 mol/LΘΜ
ΘΜΙ ¥πΑΗΈΣΘΚ0.1040 mol/LΘΜ
(6)AΘ°Υα ΫΒΈΕ®ΙήΈ¥»σœ¥ΨΆ÷±Ϋ”ΉΔ»κ¥ΐ≤β“ΚHCl»ή“ΚΜαΫΪ¥ΐ≤β“ΚœΓ ΆΘ§ΒΦ÷¬≤βΒΟ≈®Ε»ΤΪΒΆΘ§Ι AΖϊΚœΘΜ
BΘ°ΒΈΕ®«Α ΔΖ≈HCl»ή“ΚΒΡΉΕ–ΈΤΩ”Ο’τΝσΥ°œ¥ΨΜΚσΟΜ”–Η…‘ο≤ΜΜαΕ‘≤βΕ®ΫαΙϊ‘λ≥…”ΑœλΘ§Ι B≤ΜΖϊΚœΘΜ
CΘ°Φν ΫΒΈΕ®ΙήΦβΉλ≤ΩΖ÷‘ΎΒΈΕ®«Α”–Τχ≈ίΘ§ΒΈΕ®ΚσΤχ≈ίœϊ ßΘ§ΜαΒΦ÷¬±ξΉΦ“ΚΧεΜΐΤΪ¥σΘ§≤βΕ®ΫαΙϊΤΪΗΏΘ§Ι C≤ΜΖϊΚœΘΜ
DΘ°ΕΝ»ΓNaOH±ξΉΦ“Κ ±Θ§ΩΣ Φ―ω ”ΕΝ ΐΘ§ΒΈΕ®Ϋα χ ±Η© ”ΕΝ ΐΘ§ΜαΒΦ÷¬±ξΉΦ“ΚΧεΜΐΤΪ–ΓΘ§Ι DΖϊΚœΘΜΙ ¥πΑΗΈΣΘΚADΘΜ

ΓΨΧβΡΩΓΩœ¬Ν– Β―ι≤ΌΉςΙφΖΕ«“Ρή¥οΒΫ Β―ιΡΩΒΡΒΡ «
―Γœν | Β―ιΡΩΒΡ | ≤ΌΉς |
A | »Γ20.00 mL―ΈΥα | ‘Ύ25 mLΥα ΫΒΈΕ®Ιή÷–ΉΑ»κ―ΈΥαΓΘΒς’ϊ≥θ ΦΕΝ ΐΈΣ5.00 mLΚσΘ§ΫΪ Θ”ύ―ΈΥα»Ϊ≤ΩΖ≈»κΉΕ–ΈΤΩ÷– |
B | ≤βΝΩ±ΞΚΆNa2CO3»ή“ΚΒΡpH÷Β | ”Ο’τΝσΥ° Σ»σpH ‘÷ΫΘ§Ζ≈»κNa2CO3»ή“Κ÷–Θ§Ιέ≤λpH ‘÷Ϋ―’…ΪΘ§≤Δ”κ±»…ΪΩ®Ε‘±»Θ§ΕΝ≥ωpH÷Β |
C | ÷Τ»Γ¥ΩΨΜΒΡFeCl 3ΙΧΧε | Φ”»»’τΗ…FeCl3»ή“Κ |
D | ―ι÷ΛKsp[Cu(OH)2]ΘΦ Ksp[Mg(OH)2] | ΫΪ0.1 molΓΛL-1 MgSO4»ή“ΚΒΈ»κNaOH»ή“Κ÷Ν≤Μ‘Ό”–≥ΝΒμ≤ζ…ζΘ§‘ΌΒΈΦ”0.1 mol/L CuSO4»ή“Κ |
A. A B. B C. C D. D