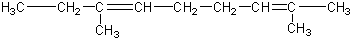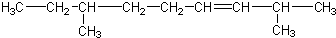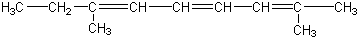��Ŀ����
7����������ʵ�У�����ʲô����Ӱ���˻�ѧ��Ӧ���ʣ���1������ƿ�������������Ļ�����壬��ƿ���ȼþ��ʱ��������屬ը���գ�
��2���ۻ���KClO3�ų��������ʺ�������������MnO2�ܿ�������������
��3��ͬŨ��ͬ������������ͬ����С��þ�������������������п�������Ӧ�ﱾ�������ʣ�
��4������ʳƷ�ױ��ʣ������첻�����������¶ȣ�
��5��ͬ����С��ʯ��ʯ�ֱ���1mol•L-1�����0.1mol•L-1�����з�Ӧ���ʲ�ͬŨ�ȣ�
���� Ӱ�컯ѧ��Ӧ���ʵ����������Ƿ�Ӧ������ʣ�����������¶ȡ�Ũ�ȡ�ѹǿ�������Լ�����ĽӴ�����ȣ�����Ӱ�컯ѧ��Ӧ���ʵ����ط�����з������жϣ��Ӷ��ó���ȷ�Ľ��ۣ�
��� �⣺��1��H2��Cl2�ڳ����²���Ӧ����ƿ���ȼþ��ʱ�Ź⣬H2��Cl2������Ӧ����Ӱ������Ϊ���գ��ʴ�Ϊ�����գ�
��2��MnO2������ã���Ӱ������Ϊ�������ʴ�Ϊ��������
��3��þ�Ļ����Ա�пǿ����Ӱ������Ϊ��Ӧ�ﱾ�������ʣ��ʴ�Ϊ����Ӧ�ﱾ�������ʣ�
��4���������¸߹�ʳƷ��ù�䣬���������µͲ�����������Ӱ������Ϊ�¶ȣ��ʴ�Ϊ���¶ȣ�
��5�������Ũ�Ȳ�ͬ��Ӧ���ʲ�ͬ����Ӱ������ΪŨ�ȣ��ʴ�Ϊ��Ũ�ȣ�
���� �����Ĺؼ���Ҫ�������Ӱ�컯ѧ��Ӧ���ʵ����أ�ֻ���������ܶ�����������ȷ���жϣ���Ŀ�ѶȲ���

��ϰ��ϵ�д�
�����Ŀ
17�������й�ʵ���������ȷ���ǣ�������
| A�� | ��ȥ��NH4��2CO3��Һ�к��е�����NH4HCO3���ʵķ����Ǽ���NaOH��Һ | |
| B�� | ������HCl��Cl2ͨ�뱥��NaHCO3��Һ�в��ܵõ����������� | |
| C�� | ������Fe2O3�Ĺ����зֱ����HCl��Һ��HI��Һ�����߷�Ӧ������ͬ | |
| D�� | ������м���������ŨHNO3���ȣ��ܵõ�ʹ����ʯ��ˮ����ǵ����壬֤���ù�����һ������CO32-��HCO3- |
18��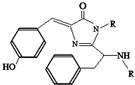 ���������ѧ��Ǯ�������ձ���ѧ�������������ѧ������•ɳ�������ڷ��ֺ��о���ɫӫ�⵰�ף�GFP����������ͻ������������2008��ŵ������ѧ�������о�����GFP�е���ɫ���Žṹ��ͼ��ʾ�������й�GFP��˵������ȷ�ģ�������
���������ѧ��Ǯ�������ձ���ѧ�������������ѧ������•ɳ�������ڷ��ֺ��о���ɫӫ�⵰�ף�GFP����������ͻ������������2008��ŵ������ѧ�������о�����GFP�е���ɫ���Žṹ��ͼ��ʾ�������й�GFP��˵������ȷ�ģ�������
 ���������ѧ��Ǯ�������ձ���ѧ�������������ѧ������•ɳ�������ڷ��ֺ��о���ɫӫ�⵰�ף�GFP����������ͻ������������2008��ŵ������ѧ�������о�����GFP�е���ɫ���Žṹ��ͼ��ʾ�������й�GFP��˵������ȷ�ģ�������
���������ѧ��Ǯ�������ձ���ѧ�������������ѧ������•ɳ�������ڷ��ֺ��о���ɫӫ�⵰�ף�GFP����������ͻ������������2008��ŵ������ѧ�������о�����GFP�е���ɫ���Žṹ��ͼ��ʾ�������й�GFP��˵������ȷ�ģ�������| A�� | ���л������ڷ����� | |
| B�� | ���л����ܷ���ˮ�ⷴӦ����1mol���л�����������������2mol | |
| C�� | ���л���������ˮ����ȡ����Ӧ����Br2 3mol | |
| D�� | ���л�����������ᷴӦ��������̼��������Һ��Ӧ |
15����֪298Kʱ��Mg��OH��2���ܶȻ�����Ksp=5.6��10-12��ȡ������MgCl2��Һ������һ�������ռ���Һ�ﵽ�����ܽ�ƽ�⣬���pH=13��������˵������ȷ���ǣ�������
| A�� | ������Һ�е�c��H+��=1.0��10-13 mol•L-1 | |
| B�� | ������Һ����ˮ���������c��OH-��=1.0��10-13 mol•L-1 | |
| C�� | ���ӵ��ռ���Һ��pH=13.0 | |
| D�� | ������Һ�е�c��Mg2+��=5.6��10-10 mol•L-1 |
2��A��B��C��D��E����ѧ��ѧ����������Ԫ�أ�ԭ����������������ṹ��������Ϣ���±���
��1��EԪ�ػ�̬ԭ�ӵĵ����Ų�ʽ��1s22s22p63s23p63d64s2��
��2���Ƚ�B��OԪ�صĵ�һ�����ܣ�B��O��A��C�ĵ縺�ԣ�A��C �����=����A D�γɵ�һ�ֳ��������������ΪҺ�壬�����õ��л��ܼ���������к��еĹ��ۼ������ǦҼ�������Ҽ����м�������
��3��д����ҵ���Ʊ�D���ʵ����ӷ���ʽ2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+2OH-+H2����
��4��E�����ں�ˮ�������绯ѧ��ʴ��д�������ĵ缫��ӦʽO2+2H2O+4e-=4OH-��
��5����һ�������£�B��D���γ�һ�ֻ�����û����������ÿ��ԭ��������Ϊ8���ӣ�������Ϊ����ɫҺ�壬��ˮǿ��ˮ�⣬�������ֲ������֮һ�ķ��ӹ���Ϊ�����Σ���һ�ֲ������Ư���ԣ�д���û�������ˮ��Ӧ�Ļ�ѧ����ʽ��NCl3+3H2O�TNH3+3HClO��
��6����֪����B����4.25g��̬�⻯��Wʱ���ͷų�11.55KJ��������д���÷�Ӧ���Ȼ�ѧ����ʽN2��g��+3H2��g��=2NH3��g����H=-92.4kJ/mol��
| Ԫ�� | �ṹ��������Ϣ |
| A | ��ԭ���������������ڲ��������2�� |
| B | ��̬ԭ�����������Ų�ΪnSnnPn+1 |
| C | �ǽ���Ԫ�أ��䵥��Ϊ���壬��������ȼ��ʱ������������ɫ���� |
| D | �����ڳ��¡���ѹ�������壮��̬ԭ�ӵ�M������1��δ�ɶԵ�P���� |
| E | ����A�γɵĺϽ�ΪĿ���������Ľ������� |
��2���Ƚ�B��OԪ�صĵ�һ�����ܣ�B��O��A��C�ĵ縺�ԣ�A��C �����=����A D�γɵ�һ�ֳ��������������ΪҺ�壬�����õ��л��ܼ���������к��еĹ��ۼ������ǦҼ�������Ҽ����м�������
��3��д����ҵ���Ʊ�D���ʵ����ӷ���ʽ2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+2OH-+H2����
��4��E�����ں�ˮ�������绯ѧ��ʴ��д�������ĵ缫��ӦʽO2+2H2O+4e-=4OH-��
��5����һ�������£�B��D���γ�һ�ֻ�����û����������ÿ��ԭ��������Ϊ8���ӣ�������Ϊ����ɫҺ�壬��ˮǿ��ˮ�⣬�������ֲ������֮һ�ķ��ӹ���Ϊ�����Σ���һ�ֲ������Ư���ԣ�д���û�������ˮ��Ӧ�Ļ�ѧ����ʽ��NCl3+3H2O�TNH3+3HClO��
��6����֪����B����4.25g��̬�⻯��Wʱ���ͷų�11.55KJ��������д���÷�Ӧ���Ȼ�ѧ����ʽN2��g��+3H2��g��=2NH3��g����H=-92.4kJ/mol��
19���ܹ���������������Ԫ�صĴ��ڵIJ����ǣ�������
| A�� | ����������ֱ�Ӽ���AgNO3��Һ | |
| B�� | ���������м�����ˮ��Ȼ�����AgNO3��Һ | |
| C�� | ���������м���NaOH��Һ�����Ⱥ��ữ��Ȼ�����AgNO3��Һ | |
| D�� | ���������м����Ҵ����Ⱥ�Ȼ�����AgNO3��Һ |
16������˵����ȷ���ǣ�������
| A�� | C5H12��ͬ���칹����Ŀ��ױ���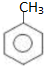 ��һ�ȴ������Ŀ��ͬ ��һ�ȴ������Ŀ��ͬ | |
| B�� | CH2�TCH2+H20$\stackrel{һ������}{��}$ CH3CH2OH ���ڻ�ԭ��Ӧ | |
| C�� | C3H6Cl2��4��ͬ���칹�壨�����������칹�� | |
| D�� | ʯ�ͷ����ú�ĸ�������������仯 |
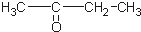 ������ͪ��
������ͪ��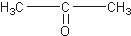 ���������ᣨ
���������ᣨ �����ߵĻ��������Ľṹ��ʽΪ��������
�����ߵĻ��������Ľṹ��ʽΪ��������