��Ŀ����
��8�֣���1��ij�¶�ʱ����2 L�ܱ������У�X��Y��Z�������ʵ����ʵ�����ʱ��仯��������ͼ��ʾ����ͼ�����ݷ������÷�Ӧ�Ļ�ѧ����ʽΪ��____________________________

��2�� ��Z��ʾ��0��2min�ڸ÷�Ӧ��ƽ����Ӧ����Ϊ
____________________
��3�� ijʱ��t��t��5min�����Y��Z���ߵ����ʵ���֮��Ϊ
3:1����X��ת����Ϊ_________
��4�� �������£���˵����Ӧ�Ѵﵽƽ��״̬����
a��������z���ʵ���Ũ��Ϊ0.25mol/L
b������Ӧ���淴Ӧ�����ʶ�Ϊ0
c��������X��Y��Z���ʵ���֮��Ϊ1��3��2
d��������X����������������
e. ��λʱ��������3a mol X��ͬʱ����2a mol Z

��2�� ��Z��ʾ��0��2min�ڸ÷�Ӧ��ƽ����Ӧ����Ϊ
____________________
��3�� ijʱ��t��t��5min�����Y��Z���ߵ����ʵ���֮��Ϊ
3:1����X��ת����Ϊ_________
��4�� �������£���˵����Ӧ�Ѵﵽƽ��״̬����
a��������z���ʵ���Ũ��Ϊ0.25mol/L
b������Ӧ���淴Ӧ�����ʶ�Ϊ0
c��������X��Y��Z���ʵ���֮��Ϊ1��3��2
d��������X����������������
e. ��λʱ��������3a mol X��ͬʱ����2a mol Z
��ÿ��2�֣���1��3X+Y 2Z ��2��0.05mol��L-1��min-1
2Z ��2��0.05mol��L-1��min-1
��3��30% ��4��a d
 2Z ��2��0.05mol��L-1��min-1
2Z ��2��0.05mol��L-1��min-1��3��30% ��4��a d
�����������1����ͼ����Կ�������Ӧ��X��Y�����ʵ������٣�ӦΪ��Ӧ�Z�����ʵ������࣬ӦΪ���������Ӧ���е�5minʱ����n��X��=0.6mol����n��Y��=0.2mol����n��Z��=0.4mol�����n��X������n��Y������n��Z��=3��1��2���μӷ�Ӧ�����ʵ����ʵ���֮�ȵ��ڻ�ѧ������֮�ȣ���Ӧ�ķ���ʽΪ��3X+Y
 2Z��
2Z����2����0��2min��Z�����ʵ����仯Ϊ0.2mol����Ũ�ȱ仯��Ϊ0.1mol/L���ʷ�Ӧ����Ϊ0.1mol/L��2min=0.05mol��L-1��min-1��
��3�����ݷ�Ӧ3X+Y
 2Z��������ʽ���£�
2Z��������ʽ���£�3X+Y
 2Z
2Z��ʼ���ʵ��� 1 1 0.1
�仯���ʵ��� 3x x 2x
ijʱ�����ʵ��� 1-3x 1-x 0.1+2x
���У���1-x������0.1+2x��=3��1�����x=0.1����X��ת����Ϊ0.3/1 ��100%=30%��
��4��a��������z���ʵ���Ũ��Ϊ0.25mol/L������ͼʾ��֪Z��Ũ�Ȳ��ٸı䣬���Ѵﵽƽ�⣻b������Ӧ���淴Ӧ�����ʶ�Ϊ0����Ӧֹͣ������ƽ��״̬��c��������X��Y��Z���ʵ���֮��Ϊ1��3��2������˵���Ƿ䣬��һ��ƽ�⣻d��������X���������������䣬˵���Ѵ�ƽ�⣻e. ��λʱ��������3a mol X��ͬʱ����2a mol Z ����Ϊͬһ������˵���Ƿ�ƽ�⣻��ѡad��

��ϰ��ϵ�д�
�����Ŀ
 2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯������ͼ��ʾ���ݴ��жϣ�
2CO2(g)��N2(g)�����ܱ������з����÷�Ӧʱ��c(CO2)���¶�(T)�������ı����(S)��ʱ��(t)�ı仯������ͼ��ʾ���ݴ��жϣ�

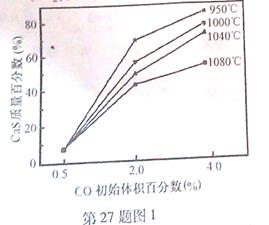
 ����������ʵ�������0.315molʱ��Ӧ�ﵽƽ�⣬����ͬ���¶��²���� ��ѹǿΪ��Ӧǰ��82.5%�������й�������ȷ����( )
����������ʵ�������0.315molʱ��Ӧ�ﵽƽ�⣬����ͬ���¶��²���� ��ѹǿΪ��Ӧǰ��82.5%�������й�������ȷ����( )
 CO2(g)��H2(g)����H��0�������¶�Խ�ߣ���v(CO)Խ��
CO2(g)��H2(g)����H��0�������¶�Խ�ߣ���v(CO)Խ�� 2Na2CO3(1)+C(s�����ʯ)����H��-1080��9kJ��mol
2Na2CO3(1)+C(s�����ʯ)����H��-1080��9kJ��mol
 CaO(s) + SO2(g) + CO2(g) ��H1=218.4kJ��mol-1(��Ӧ��)
CaO(s) + SO2(g) + CO2(g) ��H1=218.4kJ��mol-1(��Ӧ��) 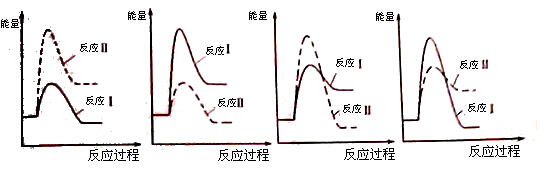

 ��
��
 ��_________���ƶ���
��_________���ƶ��� ��һ���¶��£���2 L�ݻ�������ܱ������г���4 mol
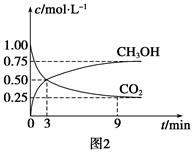
��һ���¶��£���2 L�ݻ�������ܱ������г���4 mol  ��6 mo1 H2O(g)������Ӧ��10 minʱ����Ӧ�ﵽƽ��״̬�����CH4(g)��H2(g)�����ʵ�����ʱ��仯��������ͼ��ʾ��
��6 mo1 H2O(g)������Ӧ��10 minʱ����Ӧ�ﵽƽ��״̬�����CH4(g)��H2(g)�����ʵ�����ʱ��仯��������ͼ��ʾ��
 (CO)��ʾ�Ļ�ѧ��Ӧ����Ϊ_________��
(CO)��ʾ�Ļ�ѧ��Ӧ����Ϊ_________��
 2SO3(g)����H����196.6 kJ��mol��1)����ش��������⣺
2SO3(g)����H����196.6 kJ��mol��1)����ش��������⣺
 2H2����O2��
2H2����O2�� 2H2��O2
2H2��O2 2H2����O2��
2H2����O2��