��Ŀ����
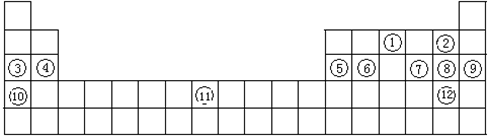
��19�֣��±���Ԫ�����ڱ���һ���֣��ش������й����⣺����дԪ�ط��Ż�ѧʽ��
|
�� ���� |
��A |
��A |
��A |
��A |
��A |
��A |
��A |
0 |
|
2 |
|
|
|
|
�� |
|
�� |
|
|
3 |
�� |
�� |
�� |
�� |
|
�� |
�� |
|
|
4[] |
�� |
|
|
|
|
|
�� |
|
(1)д������Ԫ�ط��ţ�
�� ,�� ,�� ,�� ��
(2)����ЩԪ���У�����õĽ���Ԫ���� ������õķǽ���Ԫ���� ��
��3������ЩԪ���е�����������Ӧ��ˮ�����У�������ǿ���� ��������ǿ���� �������Ե����������� ��д��ʵ������ȡ�ٵ��⻯��Ļ�ѧ����ʽ
��4������Ԫ�آ۵����ʵ���ɫ�� ����ĵ��ʵı��淽����
��5���ڢ�����У���ѧ���ʽϻ��õ��� �������û�ѧʵ��֤���������û�ѧ����ʽ˵����
�ڢ�����У���ѧ���ʻ��õ��� �������û�ѧʵ��֤���������û�ѧ����ʽ˵����
������������Ԫ�����ڱ��Ľṹ��Ԫ�������ɵ�Ӧ�á�����Ԫ�������ڱ�����λ�ÿ��жϢ���N������F������Na������Mg������Al������Si������S������Cl������K������Br��ͬ����Ԫ�����϶���ԭ�Ӱ뾶��������������ǿ���ǽ�����������ͬ����Ԫ����������ԭ�Ӱ뾶��С���ǽ���������ǿ��������������
��1��N Si S Cl ��2��K F ��3��HClO4 KOH Al��OH��3
2NH4Cl+Ca��OH��2  CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O
��4����ɫ����ɫ�ɼ�ƿ��ˮ��
��5��Na,
2Na+2H2O === 2Na��OH��2+H2��(��������Ҳ����)Mg+2H2O Mg��OH��2+H2��
Mg��OH��2+H2��
Cl, Cl2+2NaBr === Br2+2NaCl(��������Ҳ����)

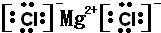
 NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ��
NH4++OH-�����ж�NH3����ˮ���γɵ�NH3?H2O�ĺ����ṹ�� ��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����
��4��1906���ŵ������ѧ������Ϊ�Ʊ�F2����������Ҫ���Ļ�ѧ��Ī��ɣ����Ԥ�����ȱ�������F2��Ӧ�Ʊ�ϡ�����廯�����Ԫ����