��Ŀ����
����Ŀ��ʵ������Ҫ0.3 mol/L NaOH��Һ480 mL��1.0 mol/L������Һ250 mL��������������Һ����������ش��������⡣
(1)��ͼ��ʾ��������������Һ�϶�����Ҫ����________(�����)������������Һ�����õ��IJ���������________(����������)��
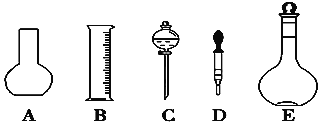
(2)���в����У�����ƿ�����߱��Ĺ�����________(�����)��
A.����һ�����ȷŨ�ȵı���Һ B.������Һ C.��������ƿ������µ����������Һ�� D.ȷϡ��ijһŨ�ȵ���Һ E.���������ܽ��������
(3)���ݼ�����������ƽ��ȡNaOH������Ϊ________g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���������ҺŨ��___0.3 mol/L(����������������������С��������ͬ)��
(4)���ݼ����֪��������������Ϊ98%���ܶ�Ϊ1.84 g/cm3��Ũ��������Ϊ_______mL(����������һλС��)�����ʵ������15 mL��20 mL��50 mL��Ͳ��Ӧѡ��________ mL����Ͳ��á�
���𰸡�AC �ձ��������� BCE 6.0 С�� 13.6 15
��������
(1) ��������һ�����ʵ���Ũ����Һ�õ�����ѡ��
(2) ����ƿ����ϡ��Ũ��Һ�����������ܽ���塢������Һ�ȣ�
(3) ����n=c��v������Ҫ���������Ƶ����ʵ�����m=n��M�����������Ƶ�����������c=![]() �����������������ܹ�ʹn������ʹV��С�IJ���������ʹcƫ�����ܹ�ʹn��С��V����IJ�������ʹcƫС��
�����������������ܹ�ʹn������ʹV��С�IJ���������ʹcƫ�����ܹ�ʹn��С��V����IJ�������ʹcƫС��
(4) ����c=![]() ����Ũ�����Ũ�ȣ��ٸ���Ũ����ϡ��ǰ�����ʵ����ʵ���������㣬����Ũ��������ѡȡ��Ͳ���
����Ũ�����Ũ�ȣ��ٸ���Ũ����ϡ��ǰ�����ʵ����ʵ���������㣬����Ũ��������ѡȡ��Ͳ���
(1) ����һ�����ʵ���Ũ����Һ�õ�������������ƽ����Ͳ��500mL����ƿ���ձ�������������ͷ�ιܡ�ҩ�ȣ���϶�����Ҫ��������AC������������Һ�����õ��IJ����������ձ�����������
(2) ����ƿΪ����������ֻ����������һ�����ȷŨ�ȵ���Һ���������ƻ��������ƿ������µ����������Һ�壬����ϡ�ͻ��ܽ�ҩƷ���������������ܽ�������ʣ������������ڴ����Һ���ʴ�Ϊ��BCE��
(3) ����0.3 mol/L NaOH��Һ480 mL����ѡ��500ml������ƿ��0.3mol/L NaOH��Һ500mL�к��е��������Ƶ����ʵ���Ϊ��0.3mol/L��0.5L=0.15mol����Ҫ�������Ƶ�����Ϊ��40g/mol��0.15mol=6.0g����ʵ����������������ȷ��������ʱ���ӿ̶��ߣ���Һ�����Vƫ������c=![]() ��֪��������ҺŨ��ƫС��
��֪��������ҺŨ��ƫС��
(4) ��������Ϊ98%���ܶ�Ϊ1.84g/cm3��Ũ�����Ũ��Ϊ��c=![]() =
=![]() =18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V=1.0mol/L��0.25L����V=0.0136L=13.6ml��ѡȡ����Ͳ���Ӧ�õ��ڻ������ȡ��Һ�������ѡ15mL��Ͳ��
=18.4mol/L��Ũ����ϡ��ǰ�����ʵ����ʵ������䣬��Ũ��������ΪV������18.4mol/L��V=1.0mol/L��0.25L����V=0.0136L=13.6ml��ѡȡ����Ͳ���Ӧ�õ��ڻ������ȡ��Һ�������ѡ15mL��Ͳ��