��Ŀ����
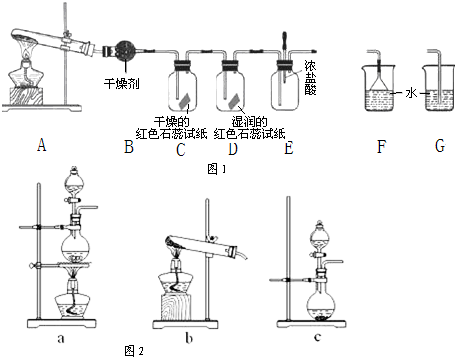
ij��ѧѧϰС��������ͼ��ʾװ����̽����Ӧ���ʵĻ�ѧ���ʡ�ѡ���ʵ��Լ����ʵ��A��B��C�����ó���Ӧ��ʵ����ۡ�ʵ��A��B��C�����Լ������ý������ڱ��С�

(1)��װ�õ����ƣ�_____________��
(2)����д�١��ݵ��Լ����ƻ�ʵ����ۣ���___________����__________����________ ����___________����______________��
(3)С�����ø�װ�����������һ��ʵ�顪��֤��NO2���������ԣ�ע����������������Na2S��Һ�����ݴ˻ش��������⣺
����μ����װ�õ������ԣ�_______��
�ڴ�װ�õĻ�����װ�����з�����Ӧ�Ļ�ѧ����ʽ��___________��
��ijͬѧ��С����ʵ�������������ɣ���Ϊ������Һ����Dz�����֤��NO2���������ԣ�����Ϊ���������ǣ�__________ ���û�ѧ��Ӧ����ʽ�ͼ�Ҫ���ֻش𣩣�
��NO2�����ж���Ӧ��NaOH��Һ���գ���д���˷�Ӧ�����ӷ���ʽ��____________��
(2)����д�١��ݵ��Լ����ƻ�ʵ����ۣ���___________����__________����________ ����___________����______________��
(3)С�����ø�װ�����������һ��ʵ�顪��֤��NO2���������ԣ�ע����������������Na2S��Һ�����ݴ˻ش��������⣺
����μ����װ�õ������ԣ�_______��
�ڴ�װ�õĻ�����װ�����з�����Ӧ�Ļ�ѧ����ʽ��___________��
��ijͬѧ��С����ʵ�������������ɣ���Ϊ������Һ����Dz�����֤��NO2���������ԣ�����Ϊ���������ǣ�__________ ���û�ѧ��Ӧ����ʽ�ͼ�Ҫ���ֻش𣩣�
��NO2�����ж���Ӧ��NaOH��Һ���գ���д���˷�Ӧ�����ӷ���ʽ��____________��
(1)��ƿ
(2)��˫��ˮ�������������Һ�� ������������Һ �������ԣ�����> ̼��>���� ����Ũ����������Һ��������������Һ�������Ȼ�粒��壨��NH4Cl���壩
(3)�ٹرշ�Һ©���Ļ������ڱ���ע��һ������ˮ�������ܲ���ˮ�У�����ƿ��һ��ʱ����ڵ��ܿڿ��������ݲ�������ȥ�ƾ��ƣ��ڵ����в���һ��ˮ����˵��װ�õ�����������
��Cu+4HNO3��Ũ�� =Cu(NO)2+2NO2��+2H2O
��3NO2+H2O=2HNO3+ NO��NO2��ˮ��Ӧ���ɵ�HNO3��һ��ǿ�����Ե��ᣬҲ�ɽ�Na2S����ʹ��Һ�����
��2NO+2OH-=NO3-+NO2-+ H2O
(2)��˫��ˮ�������������Һ�� ������������Һ �������ԣ�����> ̼��>���� ����Ũ����������Һ��������������Һ�������Ȼ�粒��壨��NH4Cl���壩
(3)�ٹرշ�Һ©���Ļ������ڱ���ע��һ������ˮ�������ܲ���ˮ�У�����ƿ��һ��ʱ����ڵ��ܿڿ��������ݲ�������ȥ�ƾ��ƣ��ڵ����в���һ��ˮ����˵��װ�õ�����������
��Cu+4HNO3��Ũ�� =Cu(NO)2+2NO2��+2H2O
��3NO2+H2O=2HNO3+ NO��NO2��ˮ��Ӧ���ɵ�HNO3��һ��ǿ�����Ե��ᣬҲ�ɽ�Na2S����ʹ��Һ�����
��2NO+2OH-=NO3-+NO2-+ H2O

��ϰ��ϵ�д�
 �߽�������ϵ�д�
�߽�������ϵ�д�
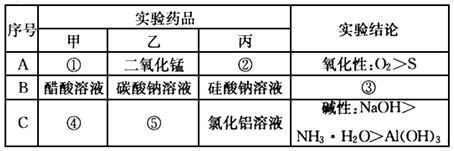
�����Ŀ
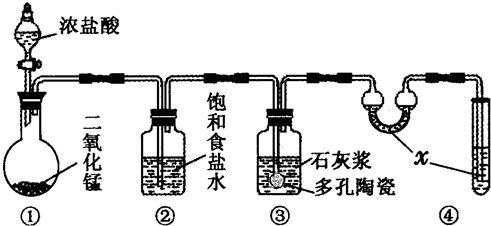
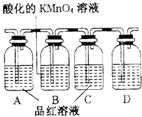 ��ѧ������ij��Ӧ�Ļ�ѧ����ʽΪA+B��C+D+H2O��δ��ƽ����Ӧ������ȥ������ش������й����⣮
��ѧ������ij��Ӧ�Ļ�ѧ����ʽΪA+B��C+D+H2O��δ��ƽ����Ӧ������ȥ������ش������й����⣮