��Ŀ����
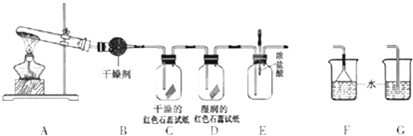
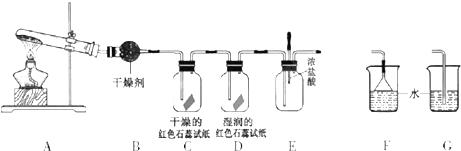
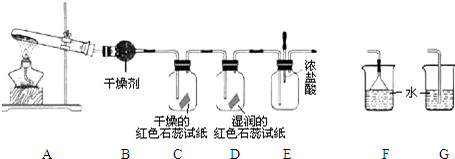
ij��ѧ��ѧ�о���ѧϰС��������ͼ1װ����ȡ��̽�����������ʣ�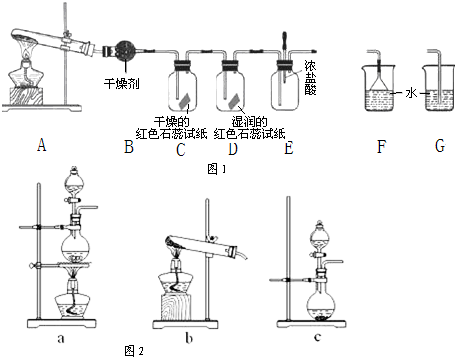
��1��A�еĻ�ѧ��Ӧ����ʽ��
��2��B�еĸ������
��3��C��Dװ������ɫ�ᷢ���仯����
��4����ʵ�����һ��ʱ���ѹEװ���еĽ�ͷ�ιܣ�����1-2��Ũ���ᣬ�ɹ۲쵽��������
��5��Ϊ��ֹ����������ɿ�����Ⱦ����Ҫ������װ�õ�ĩ������һ��β������װ�ã����ʵ�װ����
��6����ʯ����ˮ��Ӧ����Ca��OH��2���ų�������ʵ�������ô�ԭ��������ʯ���еμ�Ũ��ˮ�����Կ�����ȡ�������ô˷�����ȡ����Ӧѡ�õ����巢��װ�ã���ͼ2����
��������1������װ��A�з�����Ӧ��ʵ�����Ʊ������ķ�Ӧ�������Ȼ�狀��������ƹ�ͭ���ȷ�Ӧ���ɣ�
��2����װ���Ǽ��ȹ����Ʊ�������װ�ã������Ǽ������������
��3��������ʹʪ��ĺ�ɫʯ����ֽ������
��4��������ӷ���HCl�����ɰ��̣�
��5����Ϊ������������ˮ���������հ���ʱҪ�÷�����װ�ã�
��6�������ƹ�������ˮ��ų������ȣ�
��2����װ���Ǽ��ȹ����Ʊ�������װ�ã������Ǽ������������
��3��������ʹʪ��ĺ�ɫʯ����ֽ������
��4��������ӷ���HCl�����ɰ��̣�
��5����Ϊ������������ˮ���������հ���ʱҪ�÷�����װ�ã�
��6�������ƹ�������ˮ��ų������ȣ�
����⣺��1��A�з�����Ӧ���Ʊ������ķ�Ӧ����Ӧ�Ļ�ѧ����ʽ2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
CaCl2+2NH3��+2H2O��
��2�������Ǽ������壬�Ʊ������к���ˮ������ѡ���ʯ�Ҽ��Ը���ʴ�Ϊ����ʯ�ң�
��3��������ʹʪ��ĺ�ɫʯ����ֽ����������D����ɫ�����仯���ʴ�Ϊ��D��
��4��������ӷ���HCl�������Ȼ�茶��壬�����а����������ʴ�Ϊ�����ɴ������̣�
��5����Ϊ������������ˮ���������հ���ʱҪ�÷�����װ�ã��ʴ�Ϊ��F��
��6����ʯ������ˮ��ų������ȣ����Կ�������ʯ���еμ�Ũ��ˮ��������ȡ��������Ӧ����Ҫ���ȣ�����װ��c���ϣ��ʴ�Ϊ��c��
| ||
�ʴ�Ϊ��2NH4Cl+Ca��OH��2
| ||
��2�������Ǽ������壬�Ʊ������к���ˮ������ѡ���ʯ�Ҽ��Ը���ʴ�Ϊ����ʯ�ң�
��3��������ʹʪ��ĺ�ɫʯ����ֽ����������D����ɫ�����仯���ʴ�Ϊ��D��
��4��������ӷ���HCl�������Ȼ�茶��壬�����а����������ʴ�Ϊ�����ɴ������̣�
��5����Ϊ������������ˮ���������հ���ʱҪ�÷�����װ�ã��ʴ�Ϊ��F��
��6����ʯ������ˮ��ų������ȣ����Կ�������ʯ���еμ�Ũ��ˮ��������ȡ��������Ӧ����Ҫ���ȣ�����װ��c���ϣ��ʴ�Ϊ��c��
���������⿼���˰������Ʊ������������ʣ���Ŀ�Ѷ��еȣ����հ��������ʼ�ʵ��������������ǽ���ؼ�������������ѧ�����Ӧ����ѧ֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ
 CaCl2 + 2NH3��+ 2H2O
CaCl2 + 2NH3��+ 2H2O