��Ŀ����
 ��ѧ������ij��Ӧ�Ļ�ѧ����ʽΪA+B��C+D+H2O��δ��ƽ����Ӧ������ȥ������ش������й����⣮
��ѧ������ij��Ӧ�Ļ�ѧ����ʽΪA+B��C+D+H2O��δ��ƽ����Ӧ������ȥ������ش������й����⣮��1����A������B��ϡ���ᣨ����������A������C��Һ�У���A��C��Ӧ�����ӷ���ʽΪ
2Fe3++Fe=3Fe2+
2Fe3++Fe=3Fe2+
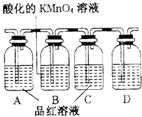
����2����C��D��Ϊ���壬�Ҷ���ʹ����ʯ��ˮ����ǣ�ij̽����ѧϰС��������ͼ������װ�����ʵ�飬֤��������Ӧ����C��D���ɣ���Bƿ��Һ��������
��ȥ���������е�SO2
��ȥ���������е�SO2
��װ��D����ʢ��Һ������ʯ��ˮ
����ʯ��ˮ
����Ҫ��֤��һ����ˮ�Ĵ��ڣ���ʹ�õ�ҩƷΪ��ˮ����ͭ
��ˮ����ͭ
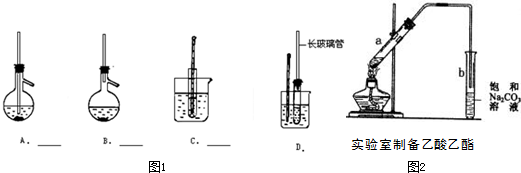
����װ��������װ���е�λ����Aǰ
Aǰ
����3����C����ɫ�д̼�����ζ�����壬��ˮ��Һ�������ԣ�д��C������������Ӧ�Ļ�ѧ����ʽ
4NH3+5O2
4NO+6H2O
| ||
| ���� |
4NH3+5O2
4NO+6H2O
��
| ||
| ���� |
��4����DΪ�ȼҵ����Ҫԭ�ϣ�C���������ЧӦ����Ҫ����֮һ���������ķ�Ӧ�Ļ�ѧ����ʽΪ
2HCl+Na2CO3=2NaCl+CO2��+H2O��HCl+NaHCO3=NaCl+CO2��+H2O
2HCl+Na2CO3=2NaCl+CO2��+H2O��HCl+NaHCO3=NaCl+CO2��+H2O
����������1��������ϡ���ᷴӦ������������һ��������ˮ��A������C��Һ�У���C�������������������ӷ�Ӧ�����������ӣ�
��2����ʹ����ʯ��ˮ����ǵ������Ƕ�������Ͷ�����̼��̼��Ũ�����ڼ��������·�Ӧ���ɶ�����������̼��ˮ������������ʹƷ����Һ��ɫ��������̼������������ʹ����ʯ��ˮ����ǣ�ע����������̼ʱҪ���ų���������ĸ��ţ������Ը��������Һ��ȥ������������ˮ����ͭ����ˮ������
��3����ˮ��Һ��������������ɫ�д̼�����ζ�������ǰ��������¡����������£�������������������һ��������ˮ��
��4���ȼҵ����Ҫԭ�����Ȼ��ƣ��������ЧӦ�������Ƕ�����̼�������̼���ƻ�̼�����Ʒ�Ӧ�������Ȼ��ơ�������̼��ˮ��
��2����ʹ����ʯ��ˮ����ǵ������Ƕ�������Ͷ�����̼��̼��Ũ�����ڼ��������·�Ӧ���ɶ�����������̼��ˮ������������ʹƷ����Һ��ɫ��������̼������������ʹ����ʯ��ˮ����ǣ�ע����������̼ʱҪ���ų���������ĸ��ţ������Ը��������Һ��ȥ������������ˮ����ͭ����ˮ������
��3����ˮ��Һ��������������ɫ�д̼�����ζ�������ǰ��������¡����������£�������������������һ��������ˮ��
��4���ȼҵ����Ҫԭ�����Ȼ��ƣ��������ЧӦ�������Ƕ�����̼�������̼���ƻ�̼�����Ʒ�Ӧ�������Ȼ��ơ�������̼��ˮ��
����⣺��1��������ϡ���ᷴӦ���������������������ӷ�Ӧ�����������ӣ��������ӷ�Ӧ����ʽΪ��2Fe3++Fe=3Fe2+��
�ʴ�Ϊ��2Fe3++Fe=3Fe2+��
��2��ʹ����ʯ��ˮ����ǵ������Ƕ�������Ͷ�����̼����Ʒ����Һ����������������Ը��������Һ���ն�������Ȼ����Ʒ����Һ������������Ƿ������Ȼ��ʣ������ͨ�����ʯ��ˮ�м��������̼������ˮ����ˮ����ͭ�������ˮ����ͭ����ɫ��֤������ˮ��A��B��C��D�ж�����ˮ������ɸ��ţ�Ϊ��ֹ���ţ�Ӧ��Aǰ���Ӹ�װ�ã�
�ʴ�Ϊ����ȥ���������е�SO2������ʯ��ˮ����ˮCuSO4��Aǰ��
��3�����´��������£������ܱ�������������һ��������ˮ����Ӧ����ʽΪ4NH3+5O2
4NO+6H2O��
�ʴ�Ϊ��4NH3+5O2
4NO+6H2O��
��4�������̼���ƻ�̼�����Ʒ�Ӧ�������Ȼ��ơ�������̼��ˮ����Ӧ����ʽΪ2HCl+Na2CO3=2NaCl+CO2��+H2O��HCl+NaHCO3=NaCl+CO2��+H2O��
�ʴ�Ϊ�������̼���ƻ�̼�����Ʒ�Ӧ�������Ȼ��ơ�������̼��ˮ��
�ʴ�Ϊ��2Fe3++Fe=3Fe2+��
��2��ʹ����ʯ��ˮ����ǵ������Ƕ�������Ͷ�����̼����Ʒ����Һ����������������Ը��������Һ���ն�������Ȼ����Ʒ����Һ������������Ƿ������Ȼ��ʣ������ͨ�����ʯ��ˮ�м��������̼������ˮ����ˮ����ͭ�������ˮ����ͭ����ɫ��֤������ˮ��A��B��C��D�ж�����ˮ������ɸ��ţ�Ϊ��ֹ���ţ�Ӧ��Aǰ���Ӹ�װ�ã�
�ʴ�Ϊ����ȥ���������е�SO2������ʯ��ˮ����ˮCuSO4��Aǰ��
��3�����´��������£������ܱ�������������һ��������ˮ����Ӧ����ʽΪ4NH3+5O2
| ||
| ���� |
�ʴ�Ϊ��4NH3+5O2
| ||
| ���� |
��4�������̼���ƻ�̼�����Ʒ�Ӧ�������Ȼ��ơ�������̼��ˮ����Ӧ����ʽΪ2HCl+Na2CO3=2NaCl+CO2��+H2O��HCl+NaHCO3=NaCl+CO2��+H2O��
�ʴ�Ϊ�������̼���ƻ�̼�����Ʒ�Ӧ�������Ȼ��ơ�������̼��ˮ��
���������⿼����Ԫ�ػ���������ʣ�ע���������Ͷ�����̼����ʹ����ʯ��ˮ����ǣ����������̼ʱҪ���ų���������ĸ��ţ�

��ϰ��ϵ�д�
 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�
�����Ŀ