��Ŀ����
����Ŀ����֪ǰ����������Ԫ��A��B��C��D��E��F��ԭ������֮��Ϊ107�������ǵĺ˵������������Bԭ�ӵ�p�������������⻯��е���ͬ��Ԫ������͵ģ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ������A��C���γ�A2C�����ӻ�������е��������������һ�����Ӳ㣬E4�����Ӻ��ԭ�ӵĺ�������Ų���ͬ����ش��������⣺
��1��A��B��C��D�ĵ�һ��������С�����˳����____________����Ԫ�ط��ţ�
��2��������BD3�ķ��ӿռ乹�Ϳ�����Ϊ_________��B��ԭ�ӹ���ӻ�����Ϊ________��
��3����֪FԪ���������ں���ƫ��ʱ����Ӱ��O2�����ڵ��������䡣��֪F2����KCN��Һ��Ӧ��F��CN��2���������������KCN��Һʱ�����ܽ⣬����������F�Ļ�̬ԭ�Ӽ۵����Ų�ʽΪ______��CN����___________��һ�ַ��ӣ���Ϊ�ȵ����壬��1��CN����������ĿΪ___________��
��4��EO2��̼�ᱵ������״̬�·�Ӧ�����þ���ľ����ṹ��ͼ��ʾ����÷�Ӧ�Ļ�ѧ����ʽΪ________
�ڸþ����У�E4��������Ϊ��Ϊ____________�����þ����߳�Ϊa nm�� ����þ�����ܶ�Ϊ__________g��cm3�������ӵ�����ΪNA��
���𰸡�Na<S<P<Cl ������ sp3 3d64s2 N2 2 TiO2+BaCO3![]() BaTiO3+CO2�� 6
BaTiO3+CO2�� 6 ![]()
��������
Bԭ�ӵ�p���������������ĵ����Ų�ʽΪ1s22s22p3��BΪNԪ�أ������⻯����˵���ṹ���Ƶ����ʣ���Է�������Խ���Ӽ���������Խ�����ʵ��۷е��Խ�ߡ���HF��H2O��NH3�ķ���֮����˴��ڷ��Ӽ��������⣬������һ�ֽ�������������������˷��Ӽ������ã�ʹ���ǵ��۷е���ͬ��Ԫ���γɵ��⻯������ߣ����ַ�������NH3�ķе���ͬ��������ߵģ����������⣬������������Ų�ʽ��1s22s22p63s23p3��BԪ��ΪPԪ�أ�����Ϊ���⻯��е���ͬ��Ԫ������͵ķ������⣬���BԪ��ΪPԪ�أ�Dԭ�ӵõ�һ�����Ӻ�3p���ȫ��������D�ĵ����Ų�ʽΪ1s22s22p63s23p5��DΪCl.Ԫ�أ�A��C���γ�A2C�����ӻ�������е��������������һ�����Ӳ㣬��A��C��Ԫ�����ڱ���λ��ͬһ���ڣ���ϻ�����ļ����ǵ�ԭ����������ClС��֪ʶ��ȷ��AΪNaԪ�أ�CΪSԪ�أ�E4�����Ӻ��ԭ�ӵĺ�������Ų���ͬ����EΪ22��Ԫ��TiԪ�أ�A��B��C��D��E��F��ԭ������֮��Ϊ107������F��ԭ������Ϊ��107-11-15-16-17-22=26��F��FeԪ�أ�������Ϸ���������������Ľ��
������Ϸ�����֪��AΪNaԪ�أ�BԪ��ΪPԪ�أ�CΪSԪ�أ�DΪClԪ�أ�EΪTiԪ�أ�F��FeԪ�أ�
��1�����ڵ��Ӳ���Խ���Ԫ�أ�ԭ�Ӻ���ĵ�����Խ�࣬ԭ�Ӱ뾶ԽС��ԭ��ʧȥ���Ӿ�Խ�ѣ��������ܾ�Խ������Na��P��S��Cl�ĵ�һ��������С�����˳����Na<S<P<Cl��
��2���ڻ�����PCl3�ķ����У�ÿ��Pԭ��������Clԭ���γ��������ۼ�����Pԭ���ϻ���һ�Թ¶Ե��ӡ�����PCl3�ķ��ӿռ乹�Ϳ�����Ϊ�����Σ�Pԭ�ӵ��ӻ���ʽΪsp3��
��3��Feԭ�ӵĻ�̬ԭ�ӵĵ����Ų�ʽΪ1s22s22p63s23p63d64s2�����̬Fe�ļ۵����Ų�ʽΪ3d64s2����������ͬ��ԭ����Ҳ��ͬ�����ͽ����ȵ����壬CN���ĵ�����Ϊ14��ԭ����Ϊ2��������CN����Ϊ�ȵ�����ķ���ΪN2���ȵ�����ṹ���ƣ�����Ҳ���ƣ�������N2��2��Nԭ�ӹ������Ե��ӣ�һ���ǦҼ���2���м���������CN���еĦм�Ҳ��2����
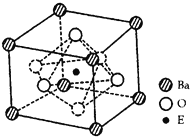
��4���ɾ����Ľṹʾ��ͼ��Ͼ�̯����֪����ÿ�������к��У�Ti��1��Ba��8��![]() =1��O:6��
=1��O:6��![]() =3�����Ըû�����Ļ�ѧʽΪBaTiO3����TiO2��̼�ᱵ������״̬�·�Ӧ�Ļ�ѧ����ʽΪTiO2+BaCO3
=3�����Ըû�����Ļ�ѧʽΪBaTiO3����TiO2��̼�ᱵ������״̬�·�Ӧ�Ļ�ѧ����ʽΪTiO2+BaCO3![]() BaTiO3+CO2�����ھ�����ÿ��Tiԭ����Χ��6��Oԭ������������ȶ����������6��Oԭ�ӹ��ɵ����������壻������λ��Ϊ6��������һ��������ֻ����һ��BaTiO3�����Ծ�����ܶ�
BaTiO3+CO2�����ھ�����ÿ��Tiԭ����Χ��6��Oԭ������������ȶ����������6��Oԭ�ӹ��ɵ����������壻������λ��Ϊ6��������һ��������ֻ����һ��BaTiO3�����Ծ�����ܶ�![]() ��
��

����Ŀ����ʽ̼����[ Cox��OH��y(CO3)z ]���������Ӳ��ϣ����Բ��ϵ����Ӽ�������ʱ�ɷֽ��������������Ϊ��ȷ������ɣ�ij��ѧ��ȤС��ͬѧ�������ͼ��ʾװ�ý���ʵ�顣
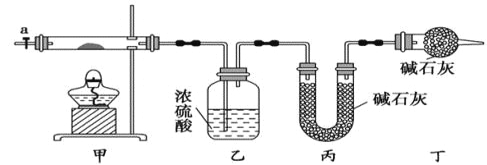
��1�����������ʵ�鲽�裺
����ȡ3.65g��Ʒ����Ӳ�ʲ������ڣ������ҡ���װ�õ�������
������ͼ��ʾװ����װ��������������װ�������ԣ�
�����ȼ��в����ܣ�����װ����____________����ʵ������ֹͣ���ȣ�
������a������ͨ����������Ӻ����ҡ���װ�õ�������
�����㡣
��2���������л���ͨ����������ӵ�Ŀ����_____________________
��3��ijͬѧ��Ϊ����ʵ��װ���д���һ������ȱ�ݣ�Ϊ�����һ���⣬��ѡ������װ���е�______(����ĸ)������_________(��װ������λ��)��
��4��������ȷװ�ý���ʵ�飬����������ݣ�
��װ�õ�����/g | ��װ�õ�����/g | |
����ǰ | 80.00 | 62.00 |
���Ⱥ� | 80.36 | 62.88 |
��ü�ʽ̼���ܵĻ�ѧʽΪ_________________��
��5������Co(AlO2)2�IJ���������ʵ���ҹ۲��Ԫ�ص���ɫ��Ӧ���ò�������ɫΪ___________��
��6��CoCl2��6H2O���������ˮ������Ӽ����Ժ��ܷ��ϣ�������Fe��Al�����ʣ���ȡCoCl2��6H2O��һ�ֹ������£�
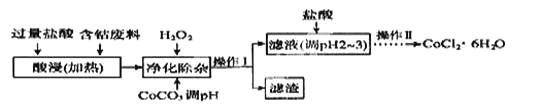
��֪��
������ | Fe(OH)3 | Fe(OH)2 | CO(OH)2 | Al(OH)2 |
��ʼ������PH�� | 2.3 | 7.5 | 7.6 | 3.4 |
��ȫ������PH�� | 4.1 | 9.7 | 9.2 | 5.2 |
����������ʱ������H2O2 ������Ӧ�����ӷ���ʽΪ______________��
������CoCO3��PHΪ5.2��7.6�����������õ������ɷ�Ϊ
�����������PHΪ2��3��Ŀ��Ϊ__________________________________��
������������Ϊ___________(���������)�����ˡ�



